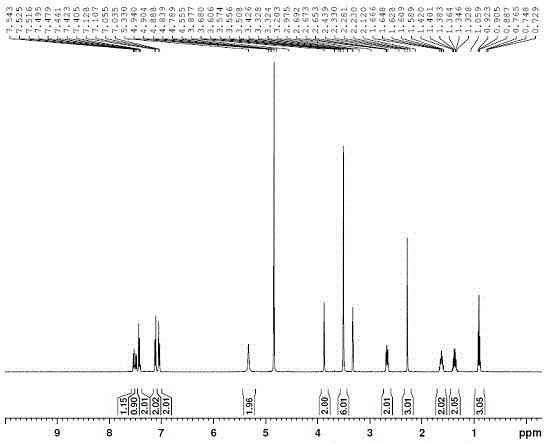
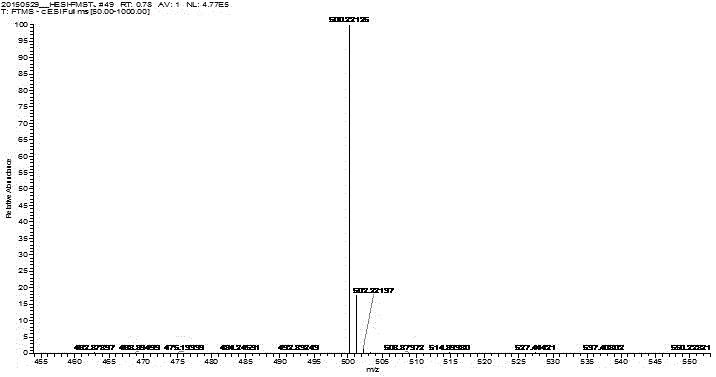
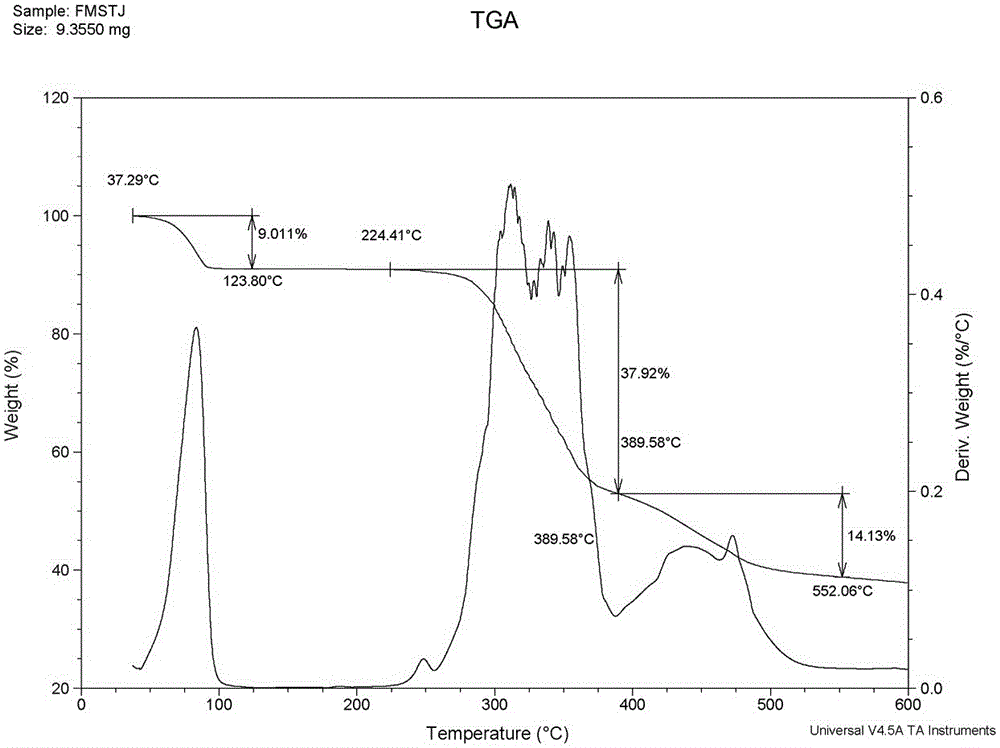
Preparation method of fimasartan potassium salt hydrate
A technology of trihydrate and Fimasartan, which is applied in the field of preparation of medicine Fimasartan potassium salt trihydrate, can solve the problems of inability to realize industrialized production, difficult to recover and apply mechanically, consume large silica gel and the like, and achieves reduction of process cost. , Simple operation, easy industrial production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of Compound IV
[0035] Starting material 1: 2-(2-Butyl-4-hydroxy-6-methylpyrimidin-5-yl)-N,N-dimethylacetamide
[0036] Starting material 2: N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium
[0037] Add 80g of starting material 1, 1000mL of ethyl acetate, and 120mL of DMF into a 2000mL reaction flask, stir and cool down to 5°C, add 3g of lithium hydride, keep stirring for 30min, add 250g of starting material 2, and heat up to 55°C , heat preservation reaction for 90h, after the heat preservation reaction, lower the temperature to 5°C, stir, a large amount of solids precipitate out, filter, wash the filter cake with ethyl acetate, and dry at 50°C to obtain 210g of off-white solid, yield: 90.6%.
[0038] (2) Preparation of compound Ⅲ
[0039] Add 210g of compound IV and 1600mL of tetrahydrofuran into a 3000mL reaction flask, add 650mL of 10% hydrochloric acid dropwise at room temperature under stirring, the solid dissolves slowly, after th...
Embodiment 2
[0047] (1) Preparation of Compound IV
[0048] Starting material 1: 2-(2-Butyl-4-hydroxy-6-methylpyrimidin-5-yl)-N,N-dimethylacetamide
[0049] Starting material 2: N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium
[0050] Add 80g of starting material 1, 1000mL of ethyl acetate, and 120mL of DMF into a 2000mL reaction flask, stir and cool down to 0°C, add 3g of lithium hydride, keep stirring for 45min, add 250g of starting material 2, and heat up to 45°C after adding , heat preservation reaction for 105h, after the heat preservation reaction, lower the temperature to 10°C, stir, a large amount of solids precipitate out, filter, wash the filter cake with ethyl acetate, and dry at 60°C to obtain 208g of off-white solid, yield: 89.7%.
[0051] (2) Preparation of compound Ⅲ
[0052] Add 210g of compound IV, 500mL of methanol, 500mL of ethanol, and 600mL of tetrahydrofuran into a 3000mL reaction flask, and add 100mL of 10% hydrochloric acid dropwise at room temperat...
Embodiment 3
[0060] (1) Preparation of Compound IV
[0061] Starting material 1: 2-(2-Butyl-4-hydroxy-6-methylpyrimidin-5-yl)-N,N-dimethylacetamide
[0062] Starting material 2: N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium
[0063] Add 80g of starting material 1, 1000mL of ethyl acetate, and 120mL of DMF into a 2000mL reaction flask, stir and cool down to 10°C, add 3g of lithium hydride, keep stirring for 15min, add 250g of starting material 2, after the addition, heat up to 65°C , heat preservation reaction for 120h, after the heat preservation reaction, lower the temperature to 0°C, stir, a large amount of solid precipitates, filter, wash the filter cake with ethyl acetate, and dry at 40°C to obtain 214g of off-white solid, yield: 92.3%.
[0064] (2) Preparation of compound Ⅲ
[0065] Add 210g of compound IV, 800mL of methanol, and 800mL of ethanol into a 3000mL reaction flask, and add 1000mL of 10% hydrochloric acid dropwise at room temperature under stirring, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dehydration rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com