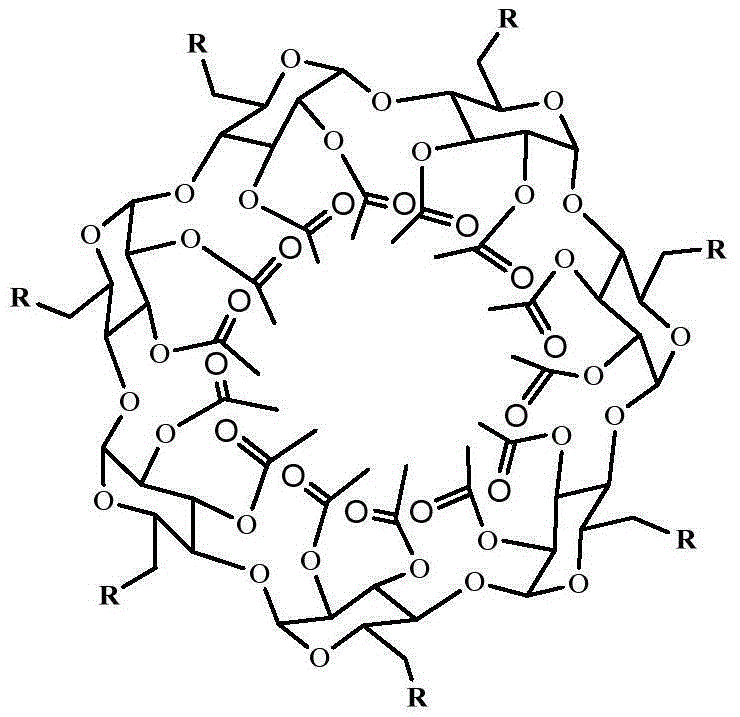
Star macromolecular antioxidant containing seven hindered phenol groups as well as preparation method and application of star macromolecular antioxidant
A technology of macromolecular antioxidants and hindered phenols, applied in the field of antioxidants, can solve the problems of unclear molecular structure and reduction of effective antioxidant components, and achieve the effect of clear molecular structure, difficult to exert, and high proportion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Synthesis of star-shaped macromolecular antioxidants containing seven hindered phenolic groups
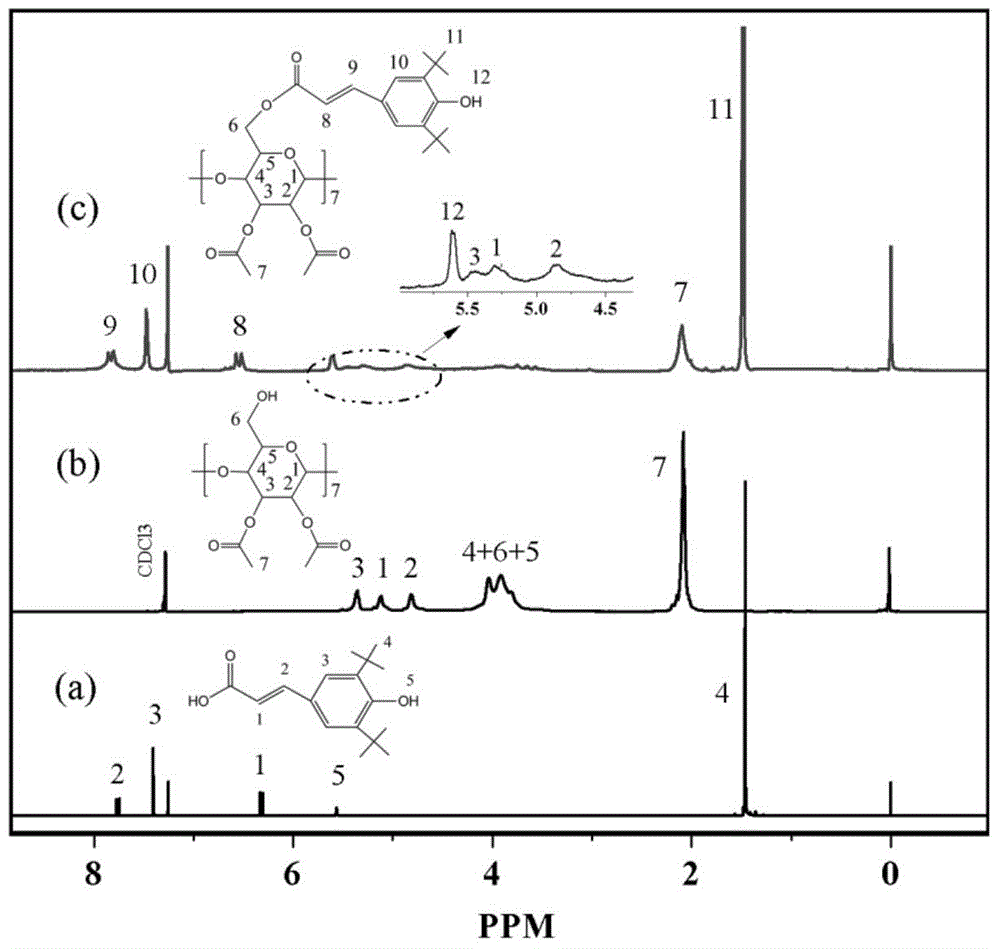
[0037] In the first step, add 4.14g of 3,5-di-tert-butyl-4-hydroxycinnamic acid (DBHCA), 20 mL of chloroform and 8 mL of Sulfone, warming up to 50°C, dripping 0.12g dimethylformamide, reacting for 5 hours, distilling under reduced pressure, removing chloroform and excess thionyl chloride, to obtain DBHCA‐Cl;
[0038] In the second step, under the protection of nitrogen, weigh 1.476gper‐2,3‐acetyl‐β‐CD, 0.04g 4‐dimethylaminopyridine (DMAP) and 2g triethylamine and dissolve them in 24mL THF. The system was lowered to 0°C and stirred; then 20mL of tetrahydrofuran was used to dissolve the first step product DBHCA‐Cl, and was added dropwise to the reaction system within 2.0 hours through a constant pressure funnel, and the temperature was raised to 30°C to continue the reaction for 48h. Rotary evaporation after the completion of the reaction to obtain a light yellow solid, w...
Embodiment 2
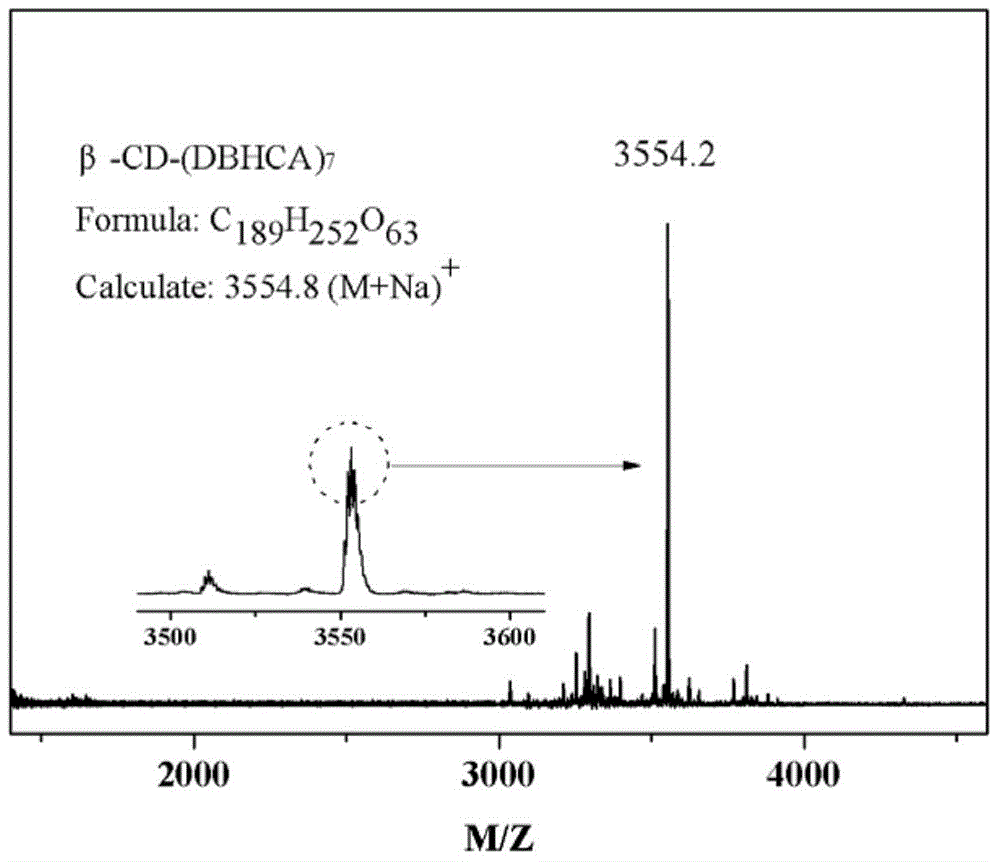
[0046] The difference between this embodiment and Example 1 is that in the first step, the consumption of thionyl chloride is changed to 12mL, and the consumption of dimethylformamide is changed to 0.16g; the reaction time is changed to 4h; the second step In the process, the amount of 4‐dimethylaminopyridine was changed to 0.08g, the amount of triethylamine was changed to 4g, and the reaction time and temperature were changed to 40°C and 24h, respectively. pass 1 H-NMR and mass spectrometry (basically the same figure 1 , 2 ) and other analyzes proved that the star-shaped macromolecular antioxidant was successfully prepared.
[0047] The thermo-oxidative aging resistance and extraction resistance of natural rubber vulcanizates are shown in Table 1. It can be seen from Table 1 that the retention of tensile strength and elongation at break of the natural rubber vulcanizate added with a star-shaped macromolecular antioxidant containing seven hindered phenolic groups after agin...
Embodiment 3
[0049] The difference between this example and Example 1 is that in the first step, the amount of chloroform is changed to 16mL, the amount of thionyl chloride is changed to 6mL, the reaction temperature is changed to 40°C, and the amount of dimethylformamide Change it into 0.08g, change the reaction time into 6h; in the second step, change the consumption of 4-dimethylaminopyridine into 0.06g, change the consumption of triethylamine into 3g, change the dropping time into 3h, change the reaction time into 72h . pass 1 H-NMR and mass spectrometry (basically the same figure 1 , 2 ) and other analyzes proved that the star-shaped macromolecular antioxidant was successfully prepared.
[0050] The thermo-oxidative aging resistance and extraction resistance of natural rubber vulcanizates are shown in Table 1. It can be seen from Table 1 that the retention of tensile strength and elongation at break of the natural rubber vulcanizate added with a star-shaped macromolecular antioxid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com