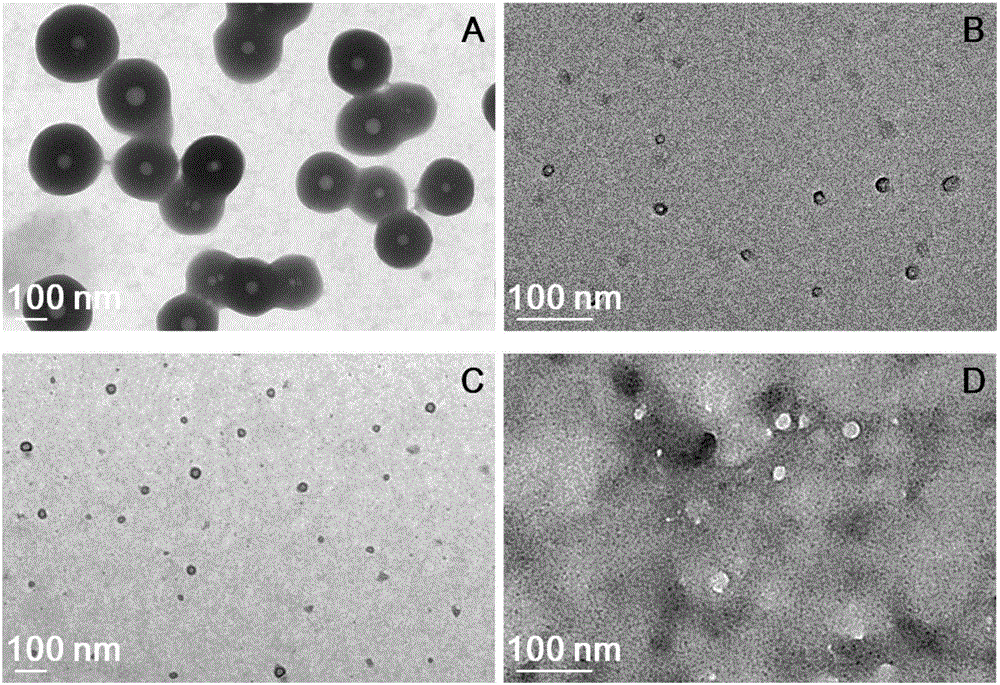
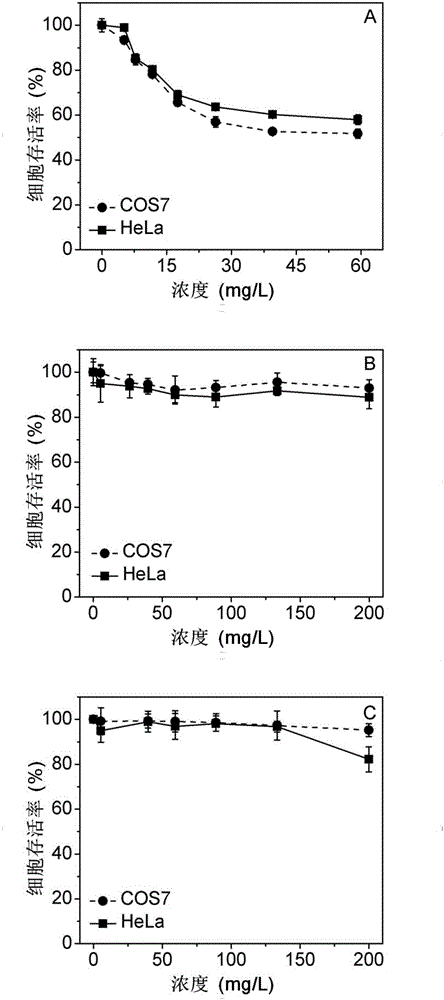
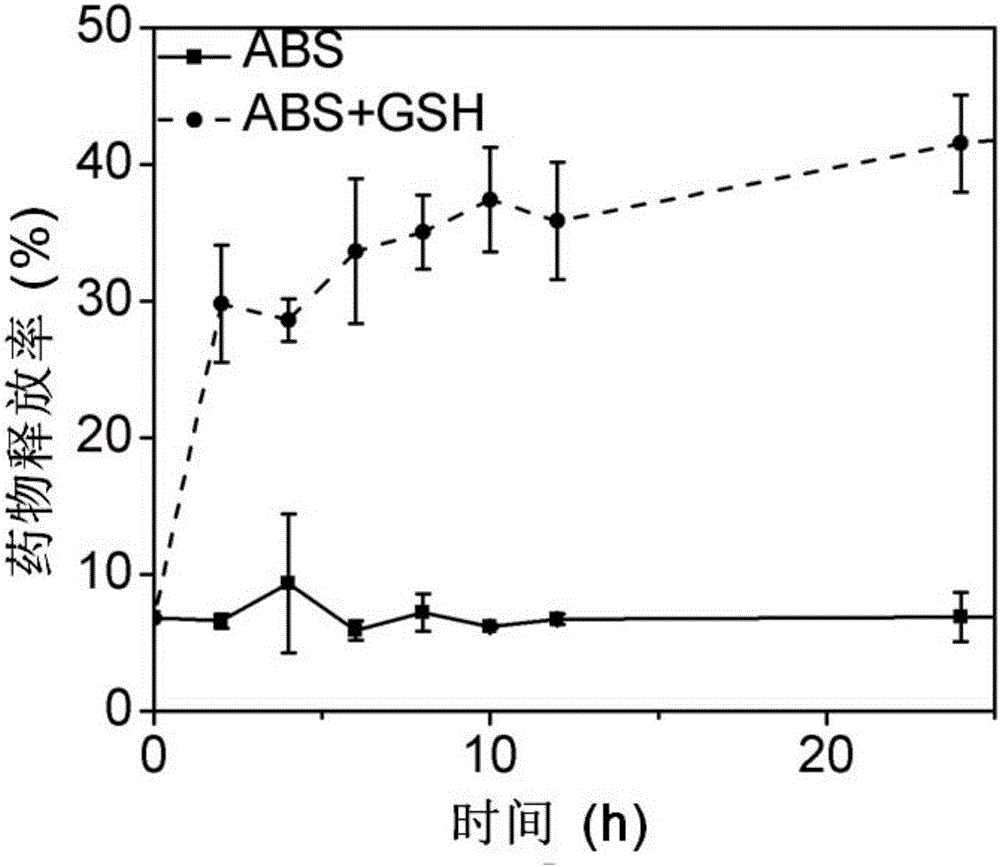
Degradable organic silicon nanocapsule drug carrier as well as preparation method and application thereof
A nanocapsule and organosilicon technology, applied in the field of biomedicine, can solve the problems of difficulty in co-carrying multiple drugs and refractory degradation, and achieve the effects of reducing multidrug resistance of tumor cells, simple operation and low equipment requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Fluorescent labeling of chitosan
[0028] Rhodamine B was used as the labeling dye, and the weight-average molecular weight was 4 × 10 using amide condensation.5 Chitosan labeling of Da. The specific preparation process is as follows: Weigh 0.479g of Rhodamine B, dissolve it in 10mL of anhydrous N-dimethylformamide (DMF), add 0.247g of N,N'-dicyclohexylcarbodiimide (DCC), N -Hydroxysuccinimide (NHS) 0.138g, reacted at 0°C for 24h in the dark. Filter and take the filtrate.
[0029] Weigh M w =4×10 5 Da chitosan 0.805g, add 80mL of water and 5mL of 1mol / L hydrochloric acid to dissolve, add the filtrate, avoid light and react at 50°C for 48h.
[0030] Add NaOH solution to adjust the pH to alkaline, precipitate chitosan, collect the precipitate by centrifugation, wash with secondary water, and centrifuge, repeating 3 times. Add 100mL of water and 5mL of 1mol / L hydrochloric acid to dissolve the chitosan after the reaction, using a molecular weight cut-off of 1.4×10 ...
Embodiment 2
[0045] (1) Fluorescent labeling of chitosan
[0046] Rhodamine B was used as the labeling dye, and the weight-average molecular weight was 2 × 10 using amide condensation. 5 Chitosan labeling of Da. The specific preparation process is as follows: Weigh 0.479g of Rhodamine B, dissolve it in 10mL of anhydrous DMF, add 0.247g of DCC and 0.138g of N-NHS, and react in the dark at 0°C for 24h. Filter and take the filtrate.
[0047] Weigh M w =2×10 5 Da chitosan 0.805g, add 80mL of water and 5mL of 1mol / L hydrochloric acid to dissolve, add the filtrate, avoid light and react at 50°C for 48h.
[0048] Add NaOH solution to adjust the pH to alkaline, precipitate chitosan, collect the precipitate by centrifugation, wash with secondary water, and centrifuge, repeating 3 times. Add 100mL of water and 5mL of 1mol / L hydrochloric acid to dissolve the chitosan after the reaction, using a molecular weight cut-off of 1.4×10 4 The dialysis bag of Da was dialyzed until the external solution ...
Embodiment 3
[0063] (1) Fluorescent labeling of chitosan
[0064] Rhodamine B was used as the labeling dye, and the weight-average molecular weight was 4 × 10 using amide condensation. 5 Chitosan labeling of Da. The specific preparation process is as follows: Weigh 0.479g of Rhodamine B, dissolve it in 10mL of anhydrous N-dimethylformamide (DMF), add 0.247g of N,N'-dicyclohexylcarbodiimide (DCC), N -Hydroxysuccinimide (NHS) 0.138g, reacted at 0°C for 24h in the dark. Filter and take the filtrate.
[0065] Weigh M w =4×10 5 Da chitosan 0.805g, add 80mL of water and 5mL of 1mol / L hydrochloric acid to dissolve, add the filtrate, avoid light and react at 50°C for 48h.
[0066] Add NaOH solution to adjust the pH to alkaline, precipitate chitosan, collect the precipitate by centrifugation, wash with secondary water, and centrifuge, repeating 3 times. Add 100mL of water and 5mL of 1mol / L hydrochloric acid to dissolve the chitosan after the reaction, using a molecular weight cut-off of 1.4×1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com