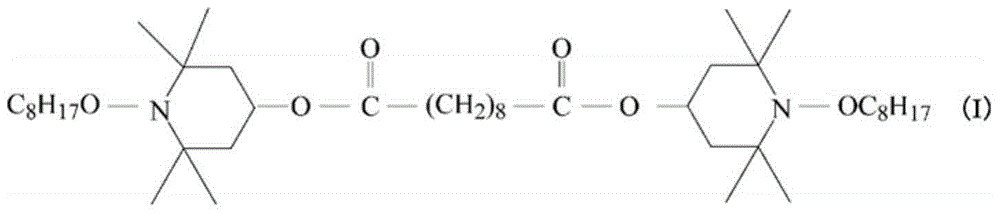Curable composition, and joint structure produced using same
A technology of curable composition and cured product, which is applied in the direction of building structure, other chemical processes, chemical instruments and methods, etc., and can solve the problem of rainwater immersion in building structures, damage to outer wall parts of the bonding interface, cracking of cured products, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
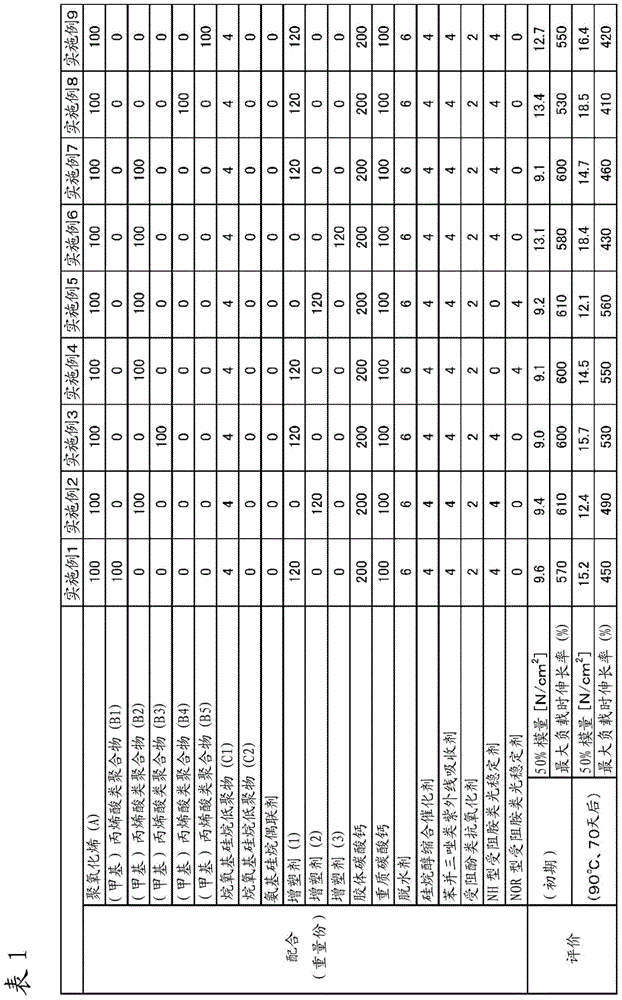
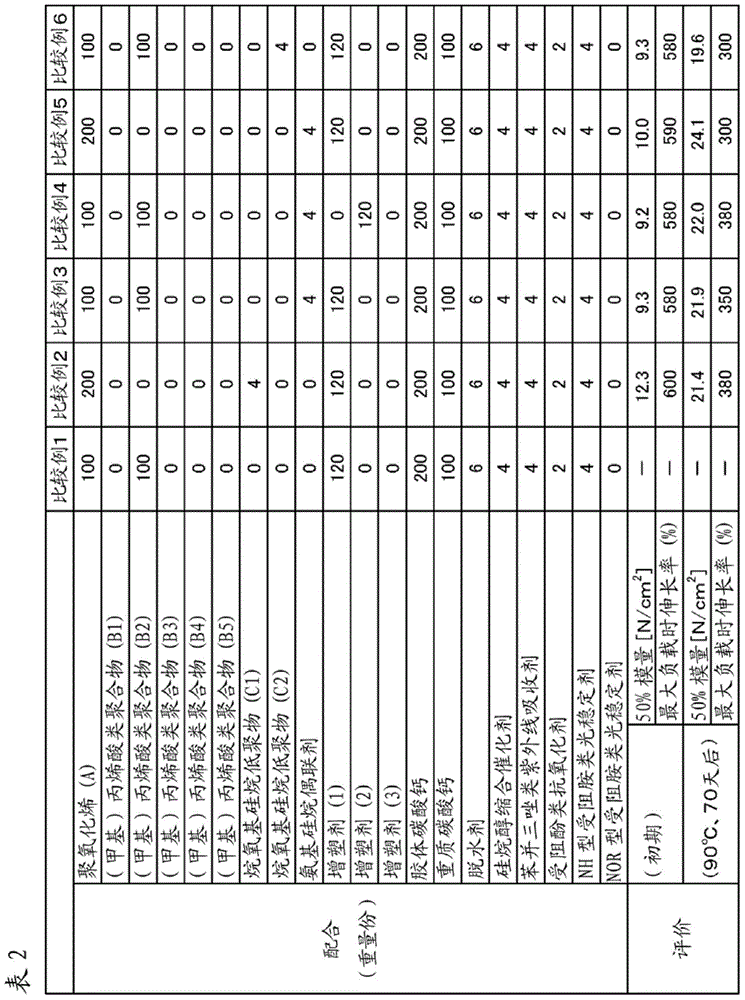
Examples
Synthetic example 1
[0113] (Synthesis Example 1: Acrylic Polymer (B1))
[0114] 100 g of n-butyl acrylate (manufactured by Nippon Catalyst Co., Ltd.), 3-methacryloyloxypropylmethyl dimethyl was supplied and mixed into a 0.5 L separable flask equipped with a stirrer, a condenser, a thermometer, and a nitrogen inlet. Oxysilane (manufactured by Shin-Etsu Chemical Co., Ltd., trade name "KBM-502") 0.6 g, 3-mercaptopropylmethyldimethoxysilane (chain transfer agent, manufactured by Shin-Etsu Chemical Co., Ltd., trade name "KBM-802") ") 0.9g and ethyl acetate 100g to make a monomer mixed solution.
[0115] Nitrogen gas was bubbled in the monomer mixed solution for 20 minutes, thereby removing dissolved oxygen in the monomer mixed solution. Next, after replacing the air in the separable flask with nitrogen, the temperature was raised to reflux while stirring the monomer mixed solution.
[0116] 0.024 g of 1,1-bis(t-hexylperoxide)-3,3,5-trimethylcyclohexane was dissolved in 1 g of ethyl acetate to prepar...
Synthetic example 2
[0123] (Synthesis Example 2: Acrylic Polymer (B4))
[0124] 100 g of n-butyl acrylate (manufactured by Nippon Catalyst Co., Ltd.), 3-methacryloyloxypropylmethyl dimethyl was supplied and mixed into a 0.5 L separable flask equipped with a stirrer, a condenser, a thermometer, and a nitrogen inlet. Oxysilane (manufactured by Shin-Etsu Chemical Co., Ltd., trade name "KBM-502") 0.9 g, 3-mercaptopropylmethyldimethoxysilane (chain transfer agent, manufactured by Shin-Etsu Chemical Co., Ltd., trade name "KBM-802") ”) 0.9g and ethyl acetate 100g to prepare a monomer mixed solution.
[0125] Polymerization was carried out in the same manner as in Synthesis Example 1 except that the above-mentioned monomer mixed solution was used. An ethyl acetate solution containing an acrylic polymer (B4) having a dimethoxymethylsilyl group was obtained.
[0126] Next, ethyl acetate was removed using an evaporator to obtain an acrylic polymer (B4). The obtained acrylic polymer (B4) had 1.85 dimethox...
Synthetic example 3
[0127] (Synthesis Example 3: Acrylic Polymer (B5))
[0128] 100 g of n-butyl acrylate (manufactured by Nippon Catalyst Co., Ltd.), 3-methacryloxypropylmethyl trimethoxyl were supplied and mixed into a 0.5 L separable flask equipped with a stirrer, a condenser, a thermometer, and a nitrogen inlet. Silane (manufactured by Shin-Etsu Chemical Co., Ltd., trade name "KBM-503") 0.6 g, 3-mercaptopropylmethyltrimethoxysilane (chain transfer agent, manufactured by Shin-Etsu Chemical Co., Ltd., trade name "KBM-803") 0.9 g and 100 g of ethyl acetate to prepare a monomer mixed solution.
[0129] Polymerization was carried out in the same manner as in Synthesis Example 1 except that the above-mentioned monomer mixed solution was used. An ethyl acetate solution containing an acrylic polymer (B4) having a dimethoxymethylsilyl group was obtained.
[0130] Next, ethyl acetate was removed using an evaporator to obtain an acrylic polymer (B5). The obtained acrylic polymer (B5) had an average of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com