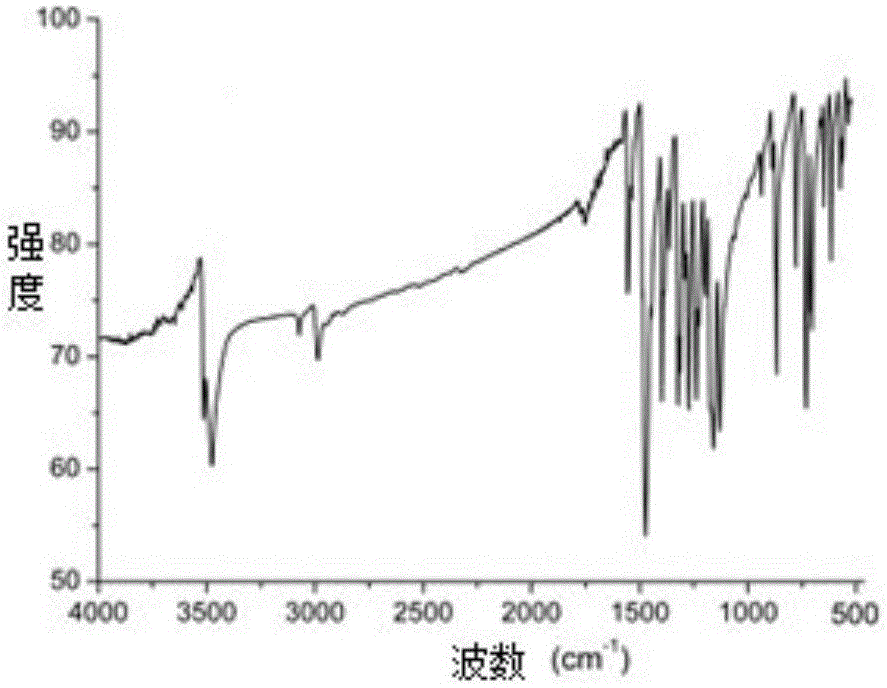
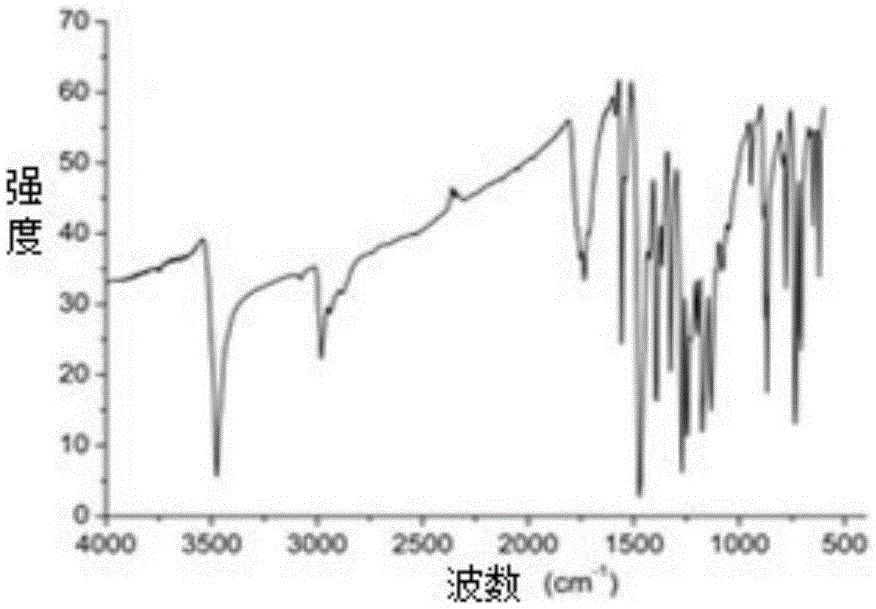
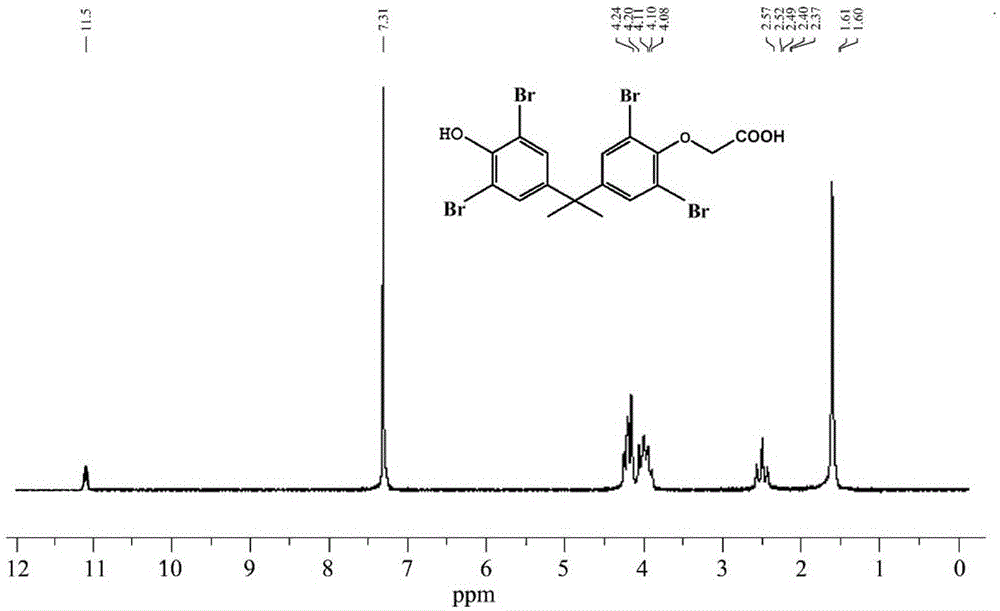
Poly-brominated biphenyl homolog semi-antigen and preparation method thereof
A technology for polybrominated biphenyls and homologues is applied in the field of hapten and its preparation, and achieves the effects of simple and feasible preparation technology, low cost and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 polybrominated biphenyl hapten
[0045] Weigh 2g (3.677mmol) TBBPA and 0.294g (7.354mmol) sodium hydroxide and dissolve in 8mL N,N-dimethylformamide, and gradually raise the temperature to 80°C. Weigh 0.5109g (3.677mmol) of bromoacetic acid and dissolve it in 5mL N,N-dimethylformamide, add it dropwise to the hot reaction solution at 80°C, and then keep the constant temperature reflux reaction for 4 to 6 hours until thin plate chromatography (TLC) until the starting point disappears. After the reaction was completed, cool to room temperature, add 30 mL of distilled water to the reaction solution, and adjust the pH to 3.0 with concentrated hydrochloric acid. At this time, a large amount of white precipitate will form; then, add 60 mL of ethyl acetate to the turbid solution, separate and save the organic layer; At this time, the organic phase was repeatedly washed with water several times to remove the reaction by-products as much as poss...
Embodiment 2
[0046] The preparation of embodiment 2 polybrominated biphenyl hapten
[0047] Weigh 2g (3.677mmol) of TBBPA and 0.588g (14.708mmol) of sodium hydroxide and dissolve in 8mL of N,N-dimethylformamide, and gradually raise the temperature to 85°C. Weigh 1.0218g (7.354mmol) of bromoacetic acid and dissolve it in 5mL of dimethyl sulfoxide, add it dropwise to the hot reaction solution at 85°C, and then keep the constant temperature reflux reaction for 4 to 6 hours until thin plate chromatography (TLC) Check until the raw material point disappears. After the reaction was completed, cool to room temperature, add 30 mL of distilled water to the reaction liquid, adjust the pH to 4.0 with concentrated hydrochloric acid, at this time, a large amount of white precipitates will form; then, add 60 mL of ethyl acetate to the cloudy liquid, separate and save the organic layer; At this time, the organic phase was repeatedly washed with water several times to remove the reaction by-products as...
Embodiment 3
[0048] The preparation of embodiment 3 polybrominated biphenyl haptens
[0049] Weigh 2g (3.677mmol) TBBPA and 0.294g (7.354mmol) sodium hydroxide and dissolve in 8mL N,N-dimethylformamide, and gradually raise the temperature to 80°C. Weigh 1.0218g (7.354mmol) of bromoacetic acid and dissolve it in 5mL of N,N-dimethylformamide, add it dropwise to the hot reaction solution at 80°C, and then maintain constant temperature and reflux for 4 to 6 hours until thin plate chromatography method (TLC) until the raw material spots disappeared. After the reaction was completed, cool to room temperature, add 30 mL of distilled water to the reaction solution, and adjust the pH to 2.0 with concentrated hydrochloric acid. At this time, a large amount of white precipitate will form; then, add 60 mL of ethyl acetate to the cloudy solution, separate and save the organic layer; At this time, the organic phase was repeatedly washed with water several times to remove the reaction by-products as m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com