Novel synthetic method for F-acrylic acid and derivative thereof
A derivative and newly synthesized technology, applied in the chemical industry, can solve the problems of expensive raw materials and high toxicity, and achieve the effect of low toxicity, simple process and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
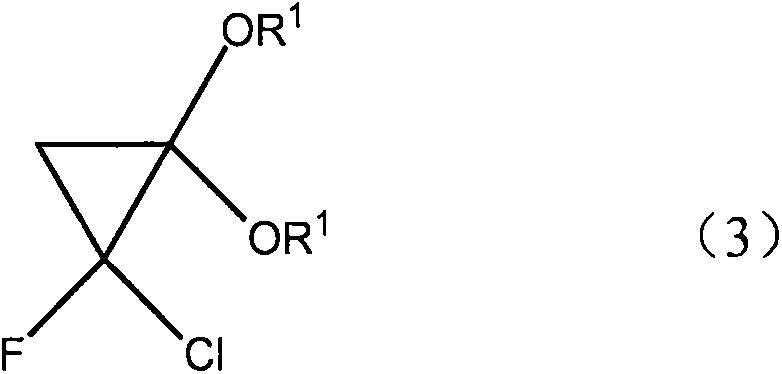
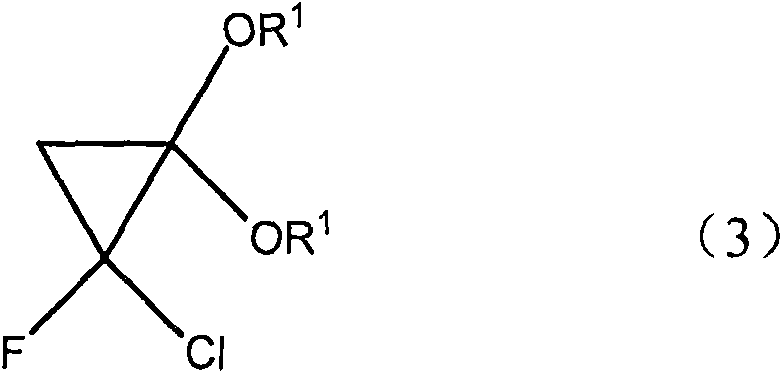
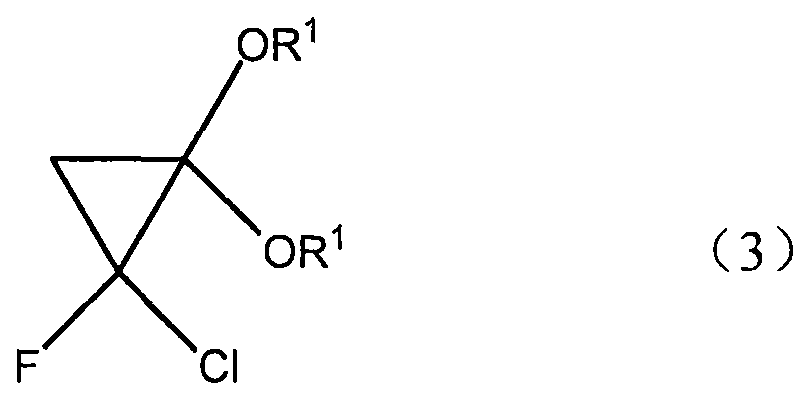
[0025] Using very cheap raw material CH 3 -C(OR 1 ) 3
[0026] Wherein R1=alkyl, aryl, pass through the ketene acetal derivative of intermediate molecular formula (2) and the cyclopropane derivative of molecular formula (3) successively, for metal oxide catalyst, what use is SiO 2 , Al2O 3 , B 2 o 3 , ZrO 2 , MgO, V 2 o 5 、TiO 2 , Fe 2 o 3 , NiO, CuO, ZnO, MoO 3 , SnO 2 , rO 2 , and their mixture, the reaction is carried out by contacting the ortho acetate of molecular formula (4) and metal oxide, the working temperature is generally at 100 to 400 ° C, preferably at 250 to 350 ° C, and the contact time is generally 1 to 120 seconds (for example, from 1 to 30 seconds), unreacted CH3-C (OR 1 ) 3 can return to the reactor to generate CH2=C(OR 1 ) 2 .
[0027] The second step is to react the ketene acetal derivative of molecular formula (2) with CHCl2F under alkaline conditions. That is, the base can be an inorganic base, such as an alkali metal hydroxide, or a...
Embodiment 2
[0030] In a stainless steel tube reactor loaded with γ-alumina, the system was heated under flowing nitrogen at 250°C for 17h. Trimethyl orthoacetate was added to the reactor under nitrogen protection at the same temperature (contact time, 10 seconds). The crude product was collected under cooling (0 °C). After washing with alkaline solution, the target product ketene acetal was collected by distillation to produce a clear liquid with a conversion rate of 70% and a selectivity of 70%.
Embodiment 3
[0032] To a mixture of ketene acetal, KOH, a phase transfer catalyst, n-hexane and water, add a n-hexane solution of fluorodichloromethane at 0°C. After stirring at the same temperature for 1 h, the layers were separated. The organic phase was washed with KOH solution, dried and distilled to obtain the target product 1-chloro-1fluoro-2,2-dimethoxycyclopropane with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com