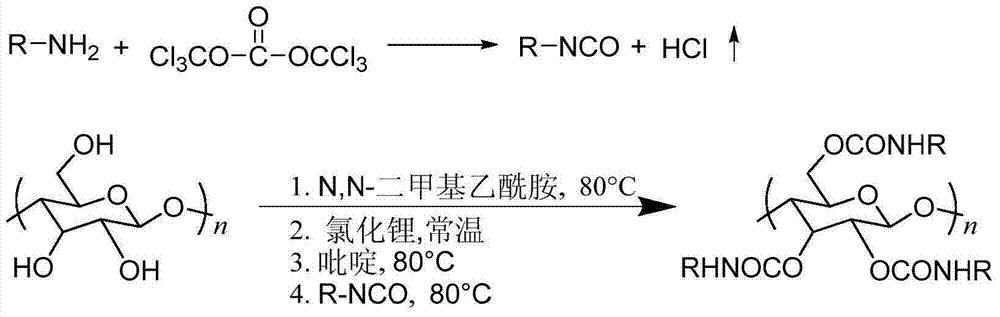
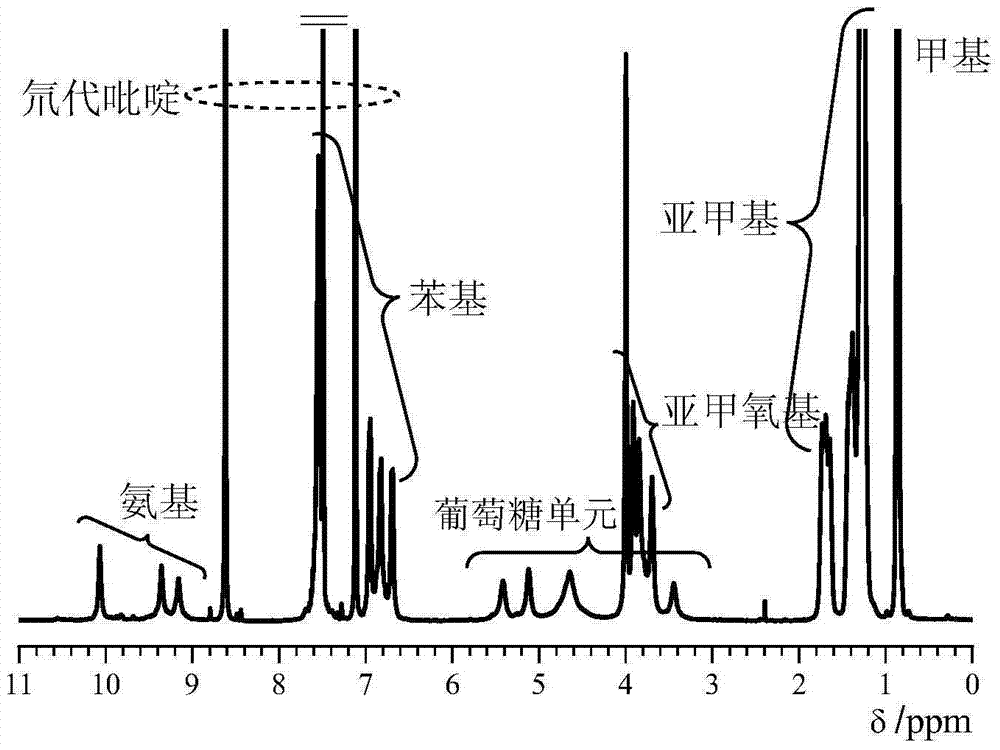
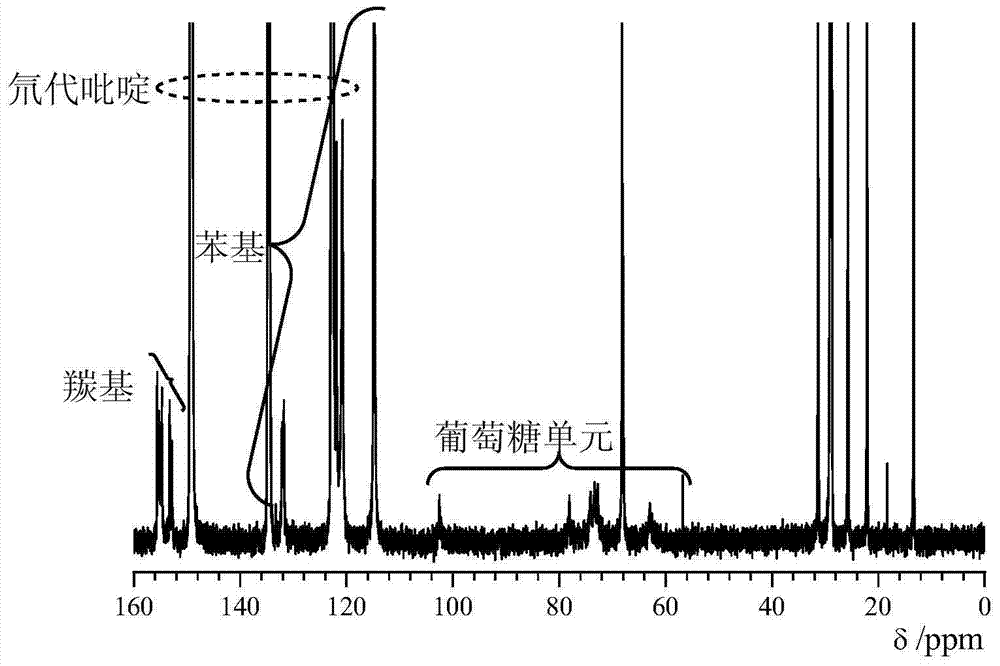
Synthetic method of cellulose derivatives with long alkoxyl side chains
A technology of cellulose and synthesis method, applied in the field of synthesis of cellulose derivatives, can solve the problems of weakening chiral recognition ability, changing secondary structure, chiral recognition ability and other performance influences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0030] 1. Dissolve 2.0 g of triphosgene in high-purity N 2Fully dissolved with an appropriate amount of ethyl acetate under protection. Transfer the pre-prepared p-octyloxyaniline solution (60% concentration) to a constant pressure low liquid funnel and wait for dropwise addition. The entire reaction system was transferred to an ice-water bath, and the low temperature environment (0-5° C.) was controlled. Complete the configuration of the triphosgene solution and the p-octyloxyaniline solution, and put them into the reaction bottle.
[0031] 2. After the reaction bottle is stabilized in the ice-water bath, start adding the aniline solution dropwise (control the dropping rate at 1 drop / 1-2s, so as not to cause the aniline concentration to be too high and aggravate the occurrence of side reactions). Stir for 20mins, transfer to a 60°C oil bath to continue the reaction. Finally, after the reaction is completed, cool down to room temperature and use high-purity N 2 Replace the...
specific Embodiment approach 2
[0034] 1. Dissolve 2.0 g of triphosgene in high-purity N 2 Fully dissolved with an appropriate amount of ethyl acetate under protection. Transfer the pre-prepared p-dodecyloxyaniline solution (60% concentration) to a constant pressure low liquid funnel and wait for dropwise addition. The entire reaction system was transferred to an ice-water bath, and the low temperature environment (0-5° C.) was controlled. Complete the configuration of the triphosgene solution and the p-dodecyloxyaniline solution, and put them into the reaction bottle.
[0035] 2. After the reaction bottle is stabilized in the ice-water bath, start adding the aniline solution dropwise (control the dropping rate at 2 drops / 1-2s, so as not to aggravate the occurrence of side reactions caused by excessive aniline concentration), after the addition is completed, continue to Stir for 30mins, transfer to a 60°C oil bath to continue the reaction. Finally, after the reaction is completed, cool down to room temper...
specific Embodiment approach 3
[0038] 1. Dissolve 2.0 g of triphosgene in high-purity N 2 Fully dissolved with an appropriate amount of ethyl acetate under protection. Transfer the pre-prepared p-hexadecyloxyaniline solution (60% concentration) to a constant pressure low liquid funnel and wait for dropwise addition. The entire reaction system was transferred to an ice-water bath, and the low temperature environment (0-5° C.) was controlled. Complete the configuration of the triphosgene solution and the hexadecyloxyaniline solution, and put them into the reaction bottle.
[0039] 2. After the reaction bottle is stabilized in the ice-water bath, start adding the aniline solution dropwise (control the dropping rate at 4 drops / 1-2s, so as not to aggravate the occurrence of side reactions caused by the high concentration of aniline). Stir for 25mins, transfer to a 60°C oil bath to continue the reaction. Finally, after the reaction is completed, cool down to room temperature and use high-purity N 2 Replace th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com