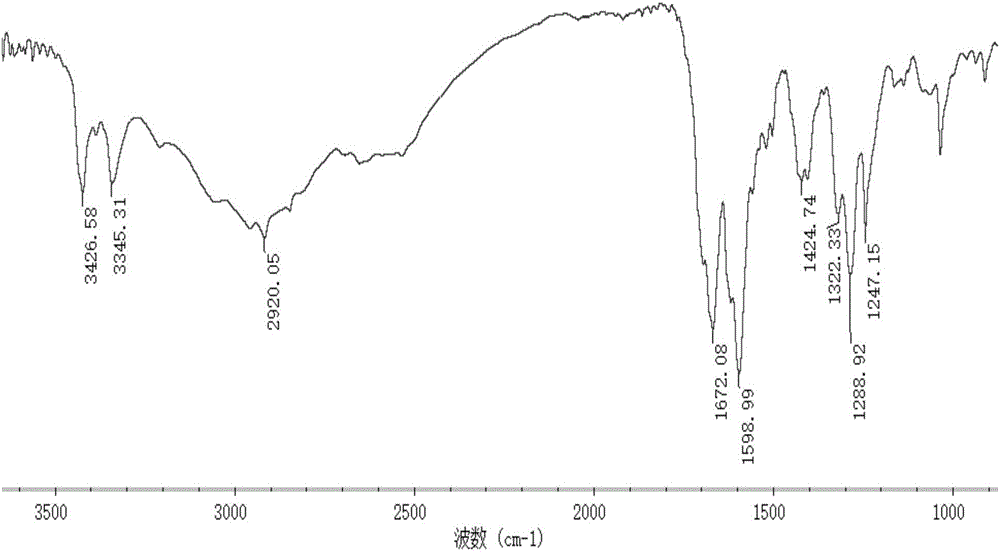
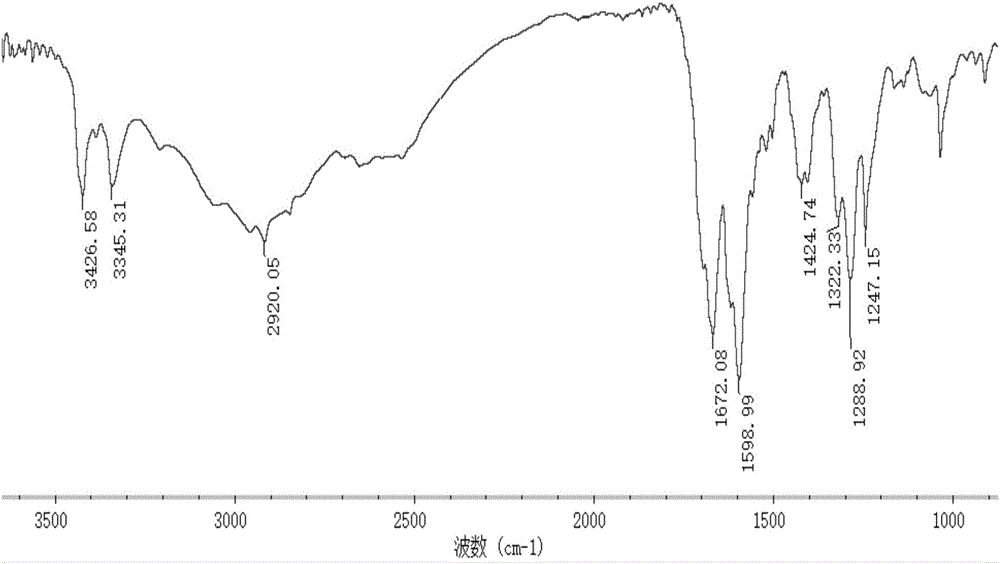
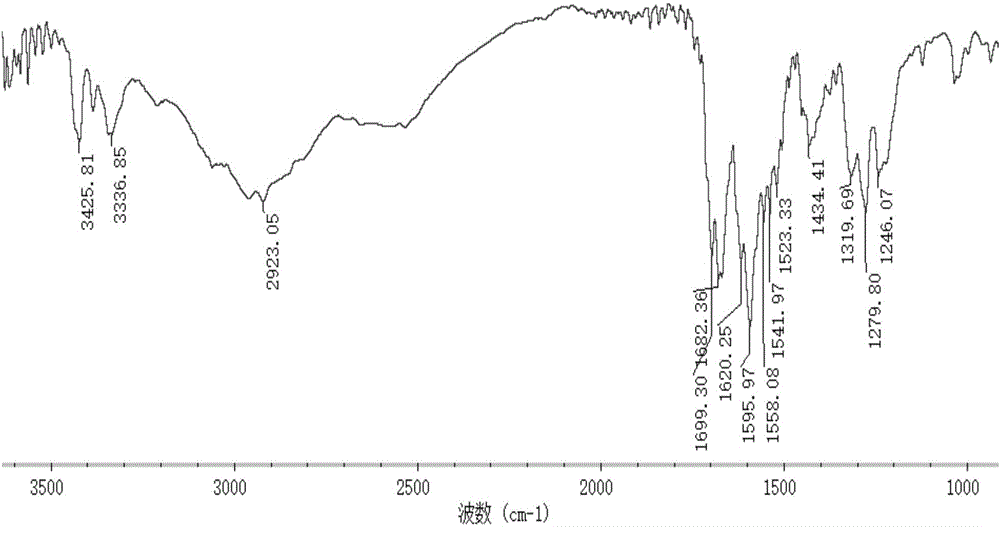
Marine organism polysaccharide copper compound, preparation thereof and application of marine organic polysaccharide copper compound serving as agricultural bactericide
A technology of marine biological polysaccharides and complexes, which is applied in the direction of chemicals, fungicides, biocides for biological control, etc., can solve the problems of high cost, difficult promotion, poor efficacy, etc. Application areas, the effect of improving antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 derivative 1
[0034] Add 2.5 grams of 4-amino-3-methylbenzoic acid and 1.66 grams of acetylacetone into 70 mL of absolute ethanol, react at 60°C for 12 hours, rotate the reactants, wash with cold ethanol, and dry at 60°C to obtain a single quarter Amine ligand.
[0035] 0.5 grams of chitosan with a molecular weight of 1.3 million was added to 20 mL of absolute ethanol and 25 mL of water, and 0.5 mL of acetic acid was added dropwise under stirring; 2.0 grams of monoquaternary ammonium ligands were added to 50 mL of absolute ethanol, and Add it to the reaction system and react at 60°C for 12 hours; add 2.5 grams of chloroacetic acid to 10mL of absolute ethanol, add dropwise to the reaction system, react at 60°C for 6 hours, cool to room temperature, add absolute ethanol to precipitate, and the precipitate is pumped Filter, wash with absolute ethanol, and dry at 60°C to obtain light yellow powder, which is O-carboxymethyl chitosan acetylac...
Embodiment 2
[0038] The preparation of embodiment 2 derivative 2
[0039] Add 2.5 grams of 4-amino-2-methylbenzoic acid and 1.66 grams of acetylacetone into 70 mL of absolute ethanol, react at 60°C for 12 hours, rotate the reactants, wash with cold ethanol, and dry at 60°C to obtain a single quaternary Amine ligand.
[0040] 0.5 grams of chitosan with a molecular weight of 1.3 million was added to 20 mL of absolute ethanol and 25 mL of water, and 0.5 mL of acetic acid was added dropwise under stirring; 2.0 grams of monoquaternary ammonium ligands were added to 50 mL of absolute ethanol, and Add it to the reaction system and react at 60°C for 12 hours; add 2.5 grams of chloroacetic acid to 10mL of absolute ethanol, add dropwise to the reaction system, react at 60°C for 6 hours, cool to room temperature, add absolute ethanol to precipitate, and the precipitate is pumped Filter, wash with absolute ethanol, and dry at 60°C to obtain light yellow powder, which is O-carboxymethyl chitosan acety...
Embodiment 3
[0043] The preparation of embodiment 3 derivative 3
[0044] Add 2.5 grams of 3-amino-2-methylbenzoic acid and 1.66 grams of acetylacetone to 70 mL of absolute ethanol, react at 60°C for 12 hours, spin evaporate the reactants, wash with cold ethanol, and dry at 60°C to obtain a single quaternary Amine ligand.
[0045] 0.5 grams of chitosan with a molecular weight of 1.3 million was added to 20 mL of absolute ethanol and 25 mL of water, and 0.5 mL of acetic acid was added dropwise under stirring; 2.0 grams of monoquaternary ammonium ligands were added to 50 mL of absolute ethanol, and Add it to the reaction system and react at 60°C for 12 hours; add 2.5 grams of chloroacetic acid to 10mL of absolute ethanol, add dropwise to the reaction system, react at 60°C for 6 hours, cool to room temperature, add absolute ethanol to precipitate, and the precipitate is pumped Filter, wash with absolute ethanol, and dry at 60°C to obtain light yellow powder, which is O-carboxymethyl chitosan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com