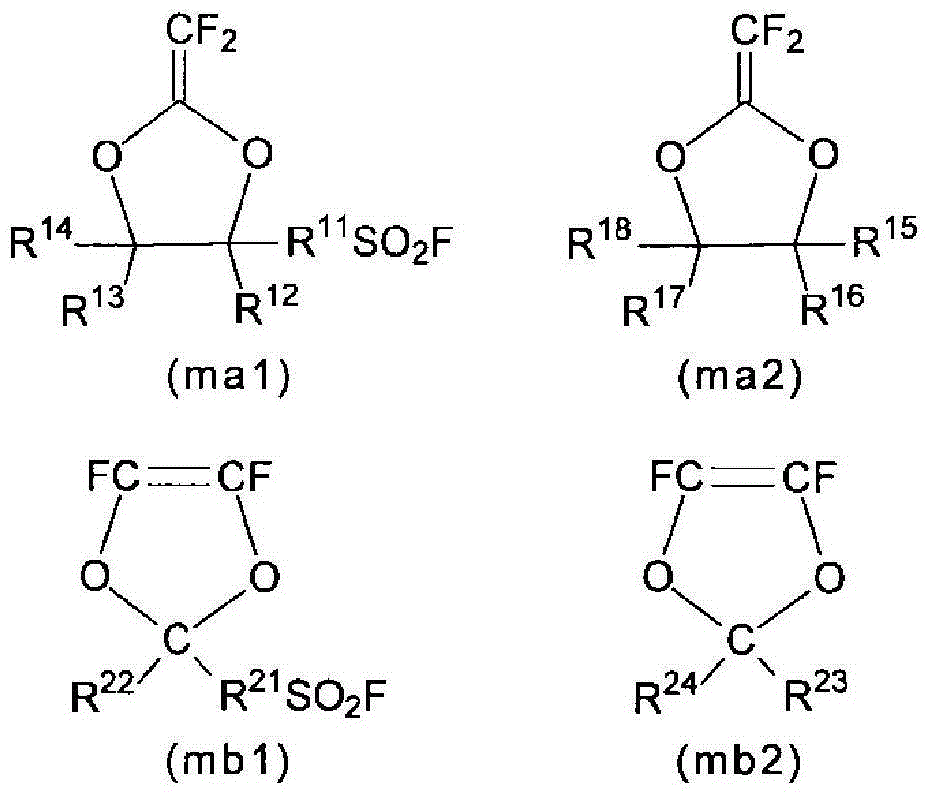Electrolyte material, liquid composition, and membrane electrode assembly for solid polymer fuel cells
A technology of electrolyte materials and compounds, applied in the direction of solid electrolyte fuel cells, fuel cells, fuel cell components, etc., can solve the problems of easy decline in power generation characteristics, large size changes, and easy flooding, etc., and achieve excellent power generation characteristics Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0183] The preparation method of the liquid composition may, for example, be a method of shearing the electrolyte material in the dispersion medium under atmospheric pressure or in a sealed state with an autoclave or the like. The preparation temperature is preferably 0 to 250°C, more preferably 20 to 150°C. If necessary, shearing such as ultrasonic waves can be applied.
[0184] The liquid composition of the present invention is suitable for forming a catalyst layer in a membrane electrode assembly described later.
[0185]
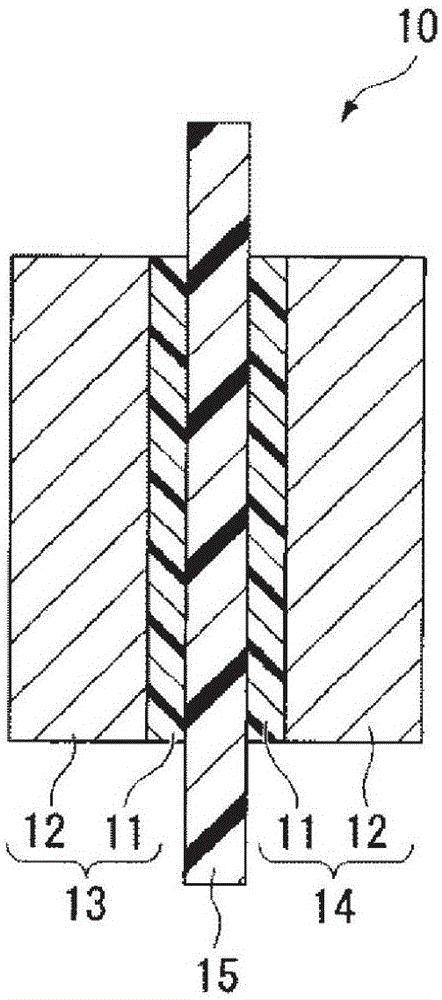
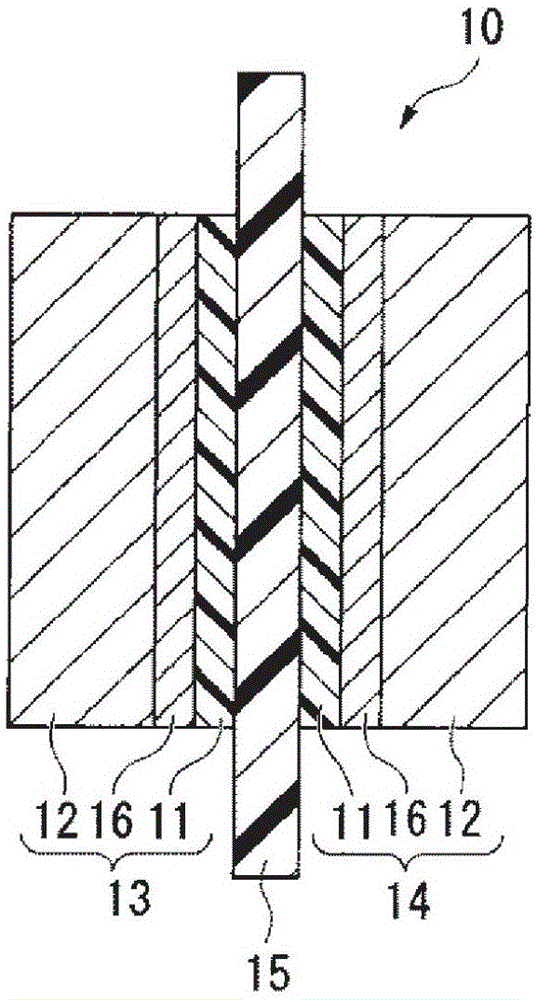
[0186] figure 1 It is a cross-sectional view showing an example of the membrane electrode assembly of the present invention. The membrane electrode assembly 10 includes an anode 13 having a catalyst layer 11 and a gas diffusion layer 12, a cathode 14 having a catalyst layer 11 and a gas diffusion layer 12, and is disposed between the anode 13 and the cathode 14 in a state of being in contact with the catalyst layer 11. Electrolyte membrane 15.
[0...
Embodiment
[0230] The following examples are given to illustrate the present invention in detail, but the present invention is not limited to these examples. Examples 1-4 are examples, and Examples 5-9 are comparative examples.
[0231] (composition of unit)
[0232] The ratio of each unit constituting the polymer (F) is determined by using 19 Compositional analysis by F-NMR was obtained.
[0233] (ion exchange capacity)
[0234] The ion exchange capacity of the polymer (H) was calculated from the ratio of each unit constituting the polymer (F).
[0235] (TQ)
[0236] TQ (unit: °C) is an indicator of the molecular weight and softening temperature of the polymer (F), and the polymer (F) is melt-extruded under the extrusion pressure of 2.94 MPa using a nozzle with a length of 1 mm and an inner diameter of 1 mm. When the extrusion volume reaches 100mm 3 / sec temperature.
[0237] Using a flow tester CFT-500D (manufactured by Shimadzu Corporation (Shimadzu Corporation)), the temperatu...
example 1
[0266] 22.47 g of compound (mb2-1), 5.10 g of compound (ma1-1), 21.10 g of compound (s-1) as a solvent, and a compound as a radical polymerization initiator were charged into a stainless steel autoclave with an inner volume of 125 mL. (i-1) 14.7 mg was fully degassed under cooling with liquid nitrogen. After the temperature was raised to 40° C., TFE was introduced, and the pressure was set at 0.40 MPaG, and the supply was continuously performed while maintaining a constant temperature and pressure. When 0.16 g of TFE was supplied, a mixture of 0.96 g of compound (mb2-1) and 1.0 g of compound (ma1-1) was supplied from a supply line cooled with dry ice. Moreover, when supplying this mixture, the supply line was cleaned using 0.5 g of compound (s-1). The supply of this mixture was performed 12 times in total. The feeding interval of this mixture is about 30 minutes. Since the supply amount of TFE reached the prescribed amount, the autoclave was cooled after 6.5 hours to termin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| carbon number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com