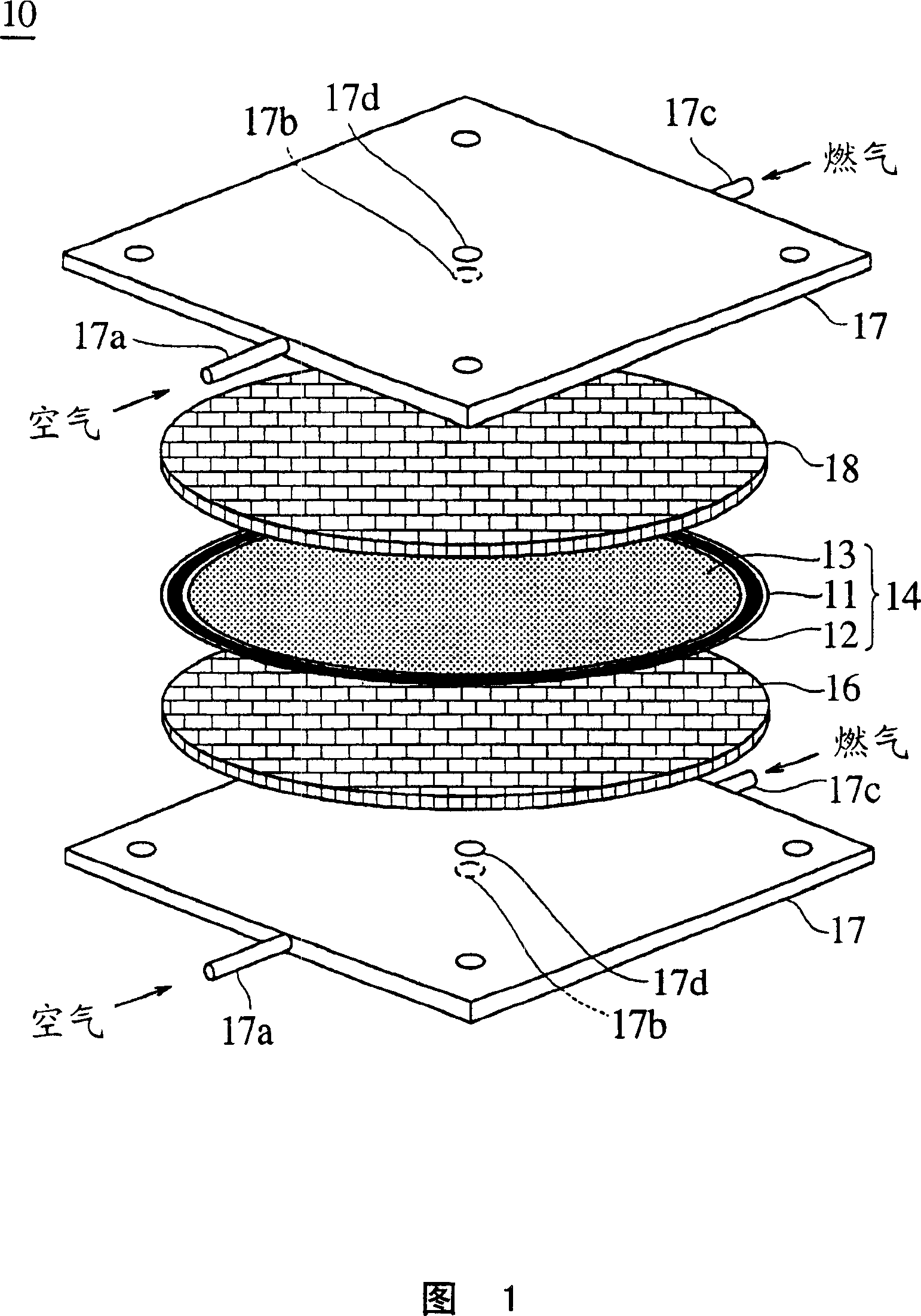
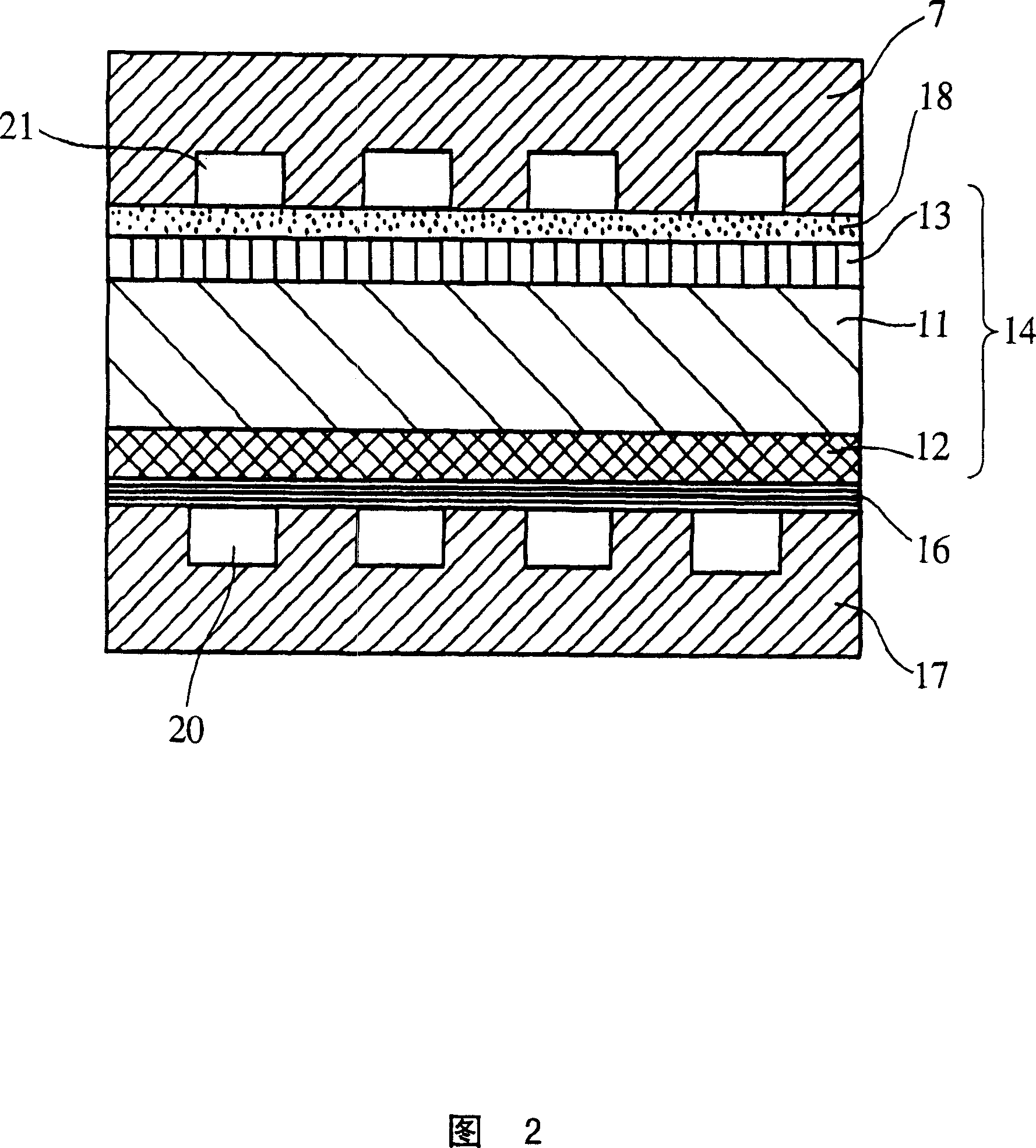
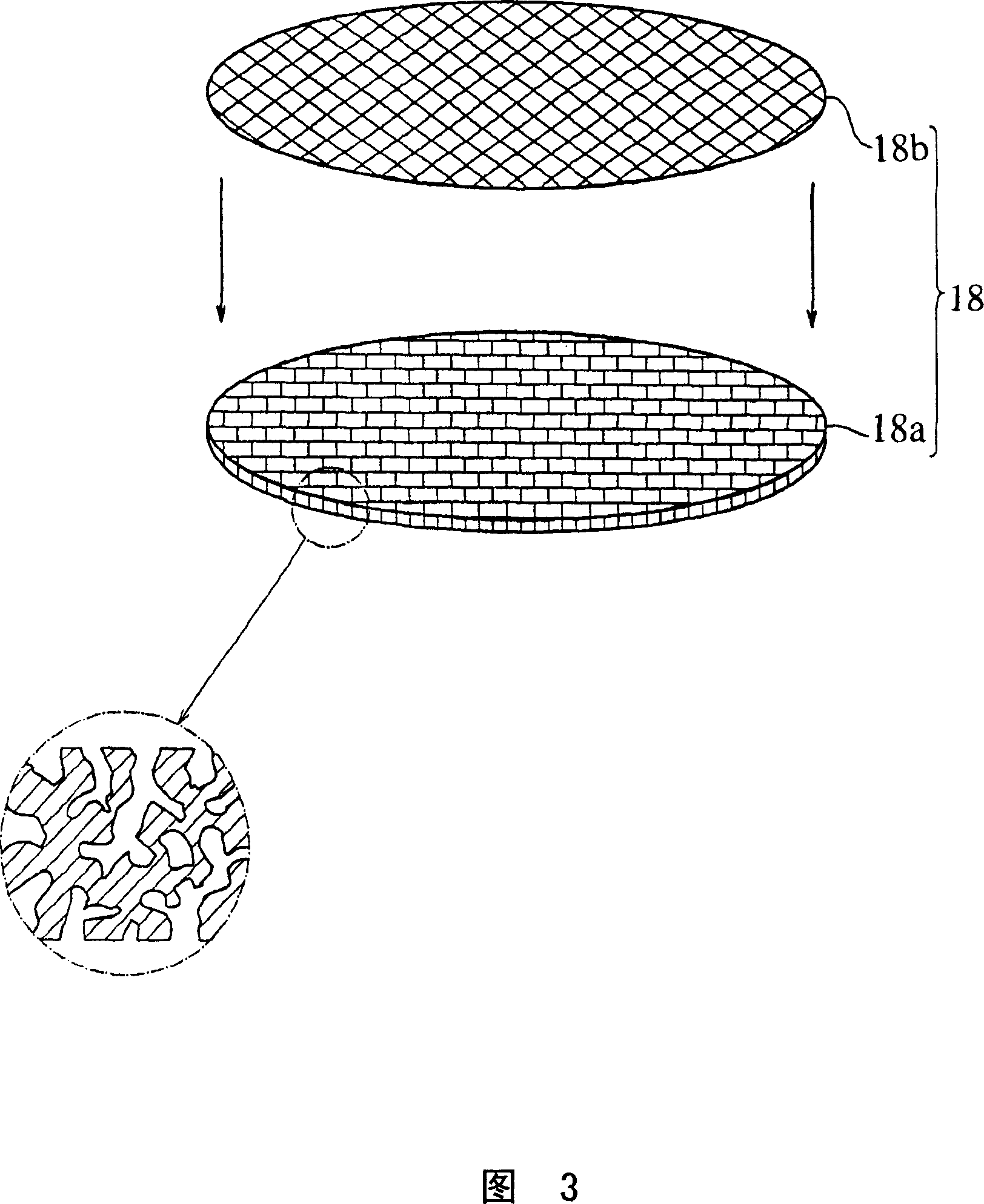
Solid electrolyte type fuel cell and air electrode current collector used for the same
A solid electrolyte and fuel cell technology, applied in solid electrolyte fuel cells, fuel cell components, fuel cells, etc., can solve the problem of high prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0076] First, pure silver atomized powder having an average particle diameter of 2 μm was prepared. The pure silver atomized powder is a powder obtained by melting pure silver in a common melting furnace and atomizing the obtained pure silver solution. And prepare n-hexane as organic solvent, sodium dodecylbenzenesulfonate (hereinafter referred to as DBS) as surfactant, hydroxypropylated cellulose methyl ether (hereinafter referred to as HPMC) as water-soluble resin binder, glycerin As plasticizer, distilled water as water.
[0077] Then, put pure silver atomized powder and HPMC (water-soluble resin binder) into a strong shear mixer and stir for 30 minutes, then add 50% of the mass of distilled water that must be added for stirring. Add the remaining 50% distilled water and other additives—n-hexane (organic solvent), DBC (surfactant) and glycerin (plasticizer) and stir for 3 hours to make a mass ratio of 50.0% pure silver atomized powder , n-hexane 1.5%, HPMC 5.0%, DBS 2.0%,...
Embodiment 4~6
[0087] Except for using Sc-stabilized zirconia to form a solid electrolyte layer, the same metal separators as in Examples 1 to 3 were used, respectively, and fuel cells were produced in the same manner as in Examples 1 to 3.
[0088] Sc-stabilized zirconia was produced by the following method. to Sc 2 o 3 and ZrOCl 2 As the initial raw material, in the hydrolysis of ZrOCl 2 Monoclinic ZrO after aqueous solution 2 A given amount of Sc was added to the sol 2 o 3 As an aqueous solution of nitric acid, urea was added and maintained at 90°C to uniformly precipitate, and the precipitate was calcined at 600°C. Sc-stabilized zirconia was obtained by sintering the calcined body at 1400° C. for one hour.
Embodiment 7~9
[0092] In addition to using 8% Y 2 o 3 Doped with ZrO 2 powder and use Y-stabilized zirconia to form a solid electrolyte layer, respectively adopt the same metal separators as in Examples 1-3, and manufacture fuel cells in the same manner as in Examples 1-3.
[0093] Y-stabilized zirconia was produced by the following method. to Y 2 o 3 and ZrOCl 2 As the initial raw material, after adding water to decompose ZrOCl 2 Monoclinic ZrO after aqueous solution 2 A given amount of Y was added to the sol 2 o 3 As an aqueous solution of nitric acid, urea was added and maintained at 90°C to uniformly precipitate, and the precipitate was calcined at 600°C. Y-stabilized zirconia was obtained by sintering the calcined body at 1400° C. for one hour.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com