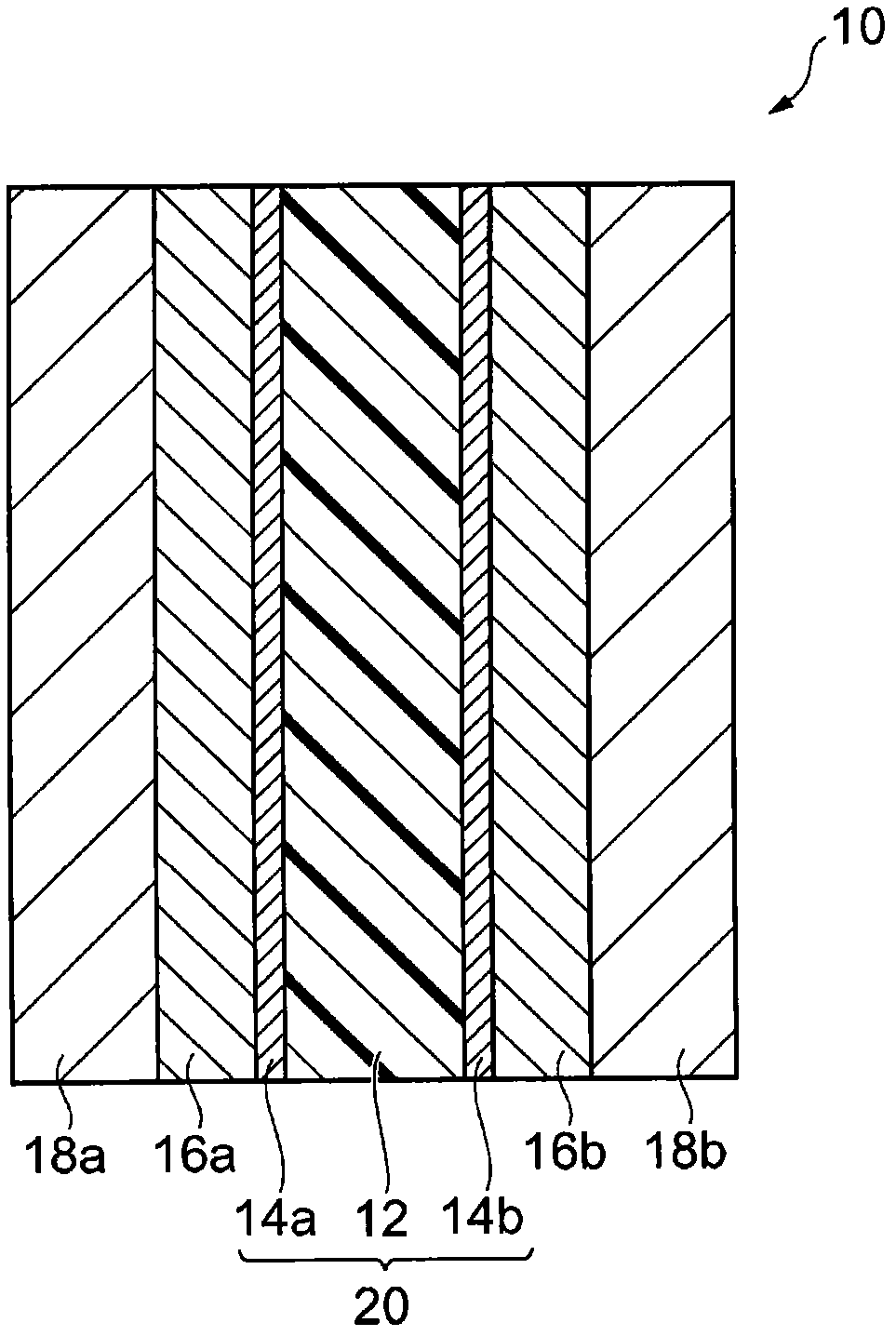
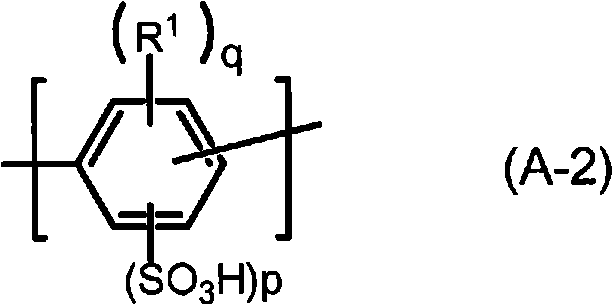Polymer, polyarylene block copolymer, polyelectrolyte, polyelectrolyte membrane, and fuel cell
A polymer electrolyte and block copolymer technology, applied in solid electrolyte fuel cells, fuel cells, fuel cell parts, etc., can solve the problems of low heat resistance, high waste cost, low membrane strength, etc., and achieve excellent Effect of water resistance and high proton conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0385] The monomer represented by the formula (1-h) and / or the monomer represented by the formula (1-i) and the monomer represented by the formula (B-4) used in the preparation of the zero-valent transition metal complex and the polymer etc. The method of mixing the polymers shown can be a method of adding one to the other or a method of adding both to the reaction vessel at the same time. The addition can be added at one time, but it is preferable to add in small amounts at a time in consideration of exothermicity, and it is also preferable to add in the presence of a solvent. The preferred solvents at this time are as follows.
[0386] The condensation reaction is usually carried out in the presence of a solvent. Examples of such solvents include N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMAc), N-methylpyrrolidone (NMP), dimethyl sulfoxide ( DMSO), hexamethylphosphoric triamide and other aprotic polar solvents, toluene, xylene, mesitylene, benzene, n-butylbenzene ...
Embodiment
[0526] The present invention will be specifically described below based on examples, but the present invention is not limited thereto.
[0527]
[0528] The number average molecular weight (Mn) and the weight average molecular weight (Mw) of the polymer electrolyte were measured by gel permeation chromatography (GPC) under the following conditions, in terms of polystyrene.
[0529] (GPC conditions)
[0530] Measuring device: Prominence GPC system manufactured by Shimadzu Corporation
[0531] Column: TSKgel GMH manufactured by Tosoh Corporation HR-M
[0532] Column temperature: 40°C
[0533] Mobile phase solvent: DMF (containing 10mmol / dm 3 LiBr)
[0534] Solvent flow: 0.5mL / min
[0535] [Preparation of Polymer Electrolyte]
Synthetic example 1
[0537] First, in an argon atmosphere, 19.7 g (90.1 mmol) of anhydrous nickel bromide and 270 g of NMP were mixed in a flask, and the inner temperature was raised to 70° C., followed by stirring for 1 hour. This was cooled to 60°C, 15.5 g (99.1 mmol) of 2,2'-bipyridine was added, and stirred at this temperature for 30 minutes to prepare a nickel-containing solution.
[0538] Then, in an argon atmosphere, 2,5-dichlorobenzenesulfonic acid (2,2-dimethylpropyl) ester 18.0 g (60.6 mmol), 2,5-dichlorobenzophenone 7.4 g (29.5mmol), dissolved in 200g of NMP, prepare a solution at 50°C, add 11.8g (180.1mmol) of zinc powder to the solution, inject the above-mentioned nickel-containing solution into it, and heat up to 65°C for 5 hours Polymerization reaction to obtain a black polymerization solution.
[0539] The obtained polymerization solution was poured into 900 g of 8N nitric acid aqueous solution at room temperature, and stirred for 30 minutes. The crude polymer product of filterin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com