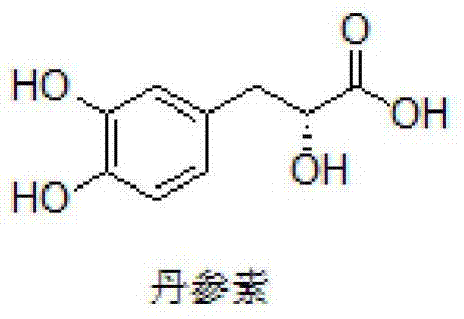
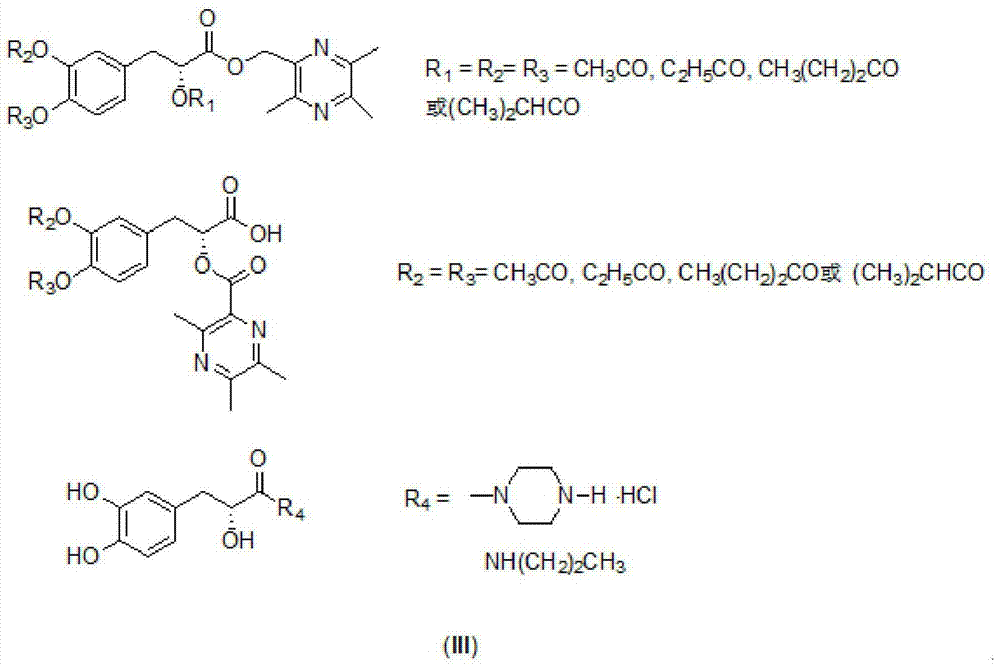
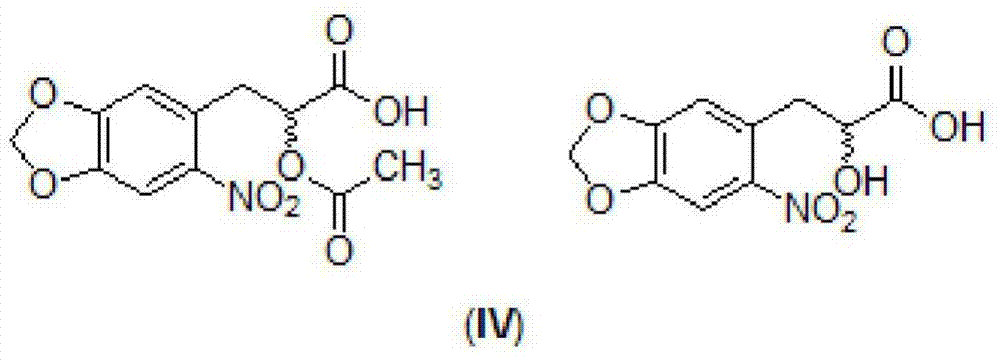
A kind of danshensu amide derivative and its preparation method and application
A technology for danshensu amide and derivatives, applied in the field of danshensu amide derivatives and their preparation, can solve the problems of few reports on structure modification, and achieve the effects of improving the permeability of blood-brain barrier, broadening applications and improving protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the preparation of starting material 2-aminomethyl-3,5,6-trimethylpyrazine
[0057]
[0058] The preparation process is as follows:
[0059]
[0060] Specific steps are as follows:
[0061] 32mL CCl 4 Added 2,3,5,6-tetramethylpyrazine 8.7g (63.7mmol) into the reaction flask, then added N-bromosuccinimide (NBS) 11.54g (64.8mmol), 23mg For benzoyl oxidation, the mixture was stirred and refluxed for 8-9 hours under the irradiation of a 200W incandescent lamp; after the reaction, the reaction solution was cooled to below 5°C, and succinimide was removed by suction filtration to obtain 13.0 g of a brown oil. The crude product was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 5:1, v / v) to obtain 8.3 g of pure 2-bromomethyl-3,5,6-trimethylpyrazine (1), It is a white to light pink low-melting solid with a yield of 60%. 1 H NMR (CDCl 3 ,300MHz):2.51(m,6H,CH 3 ),2.60(s,3H,CH 3 ),4.57(s,2H,CH 2 ).
[0062] Add 4.3g (20...
Embodiment 2
[0066] Embodiment 2: Preparation of starting material 3,5,6-trimethylpyrazine-2-carboxylic acid
[0067]
[0068] The preparation process is as follows:
[0069]
[0070] Concrete synthetic steps are as follows:
[0071] Add 2,3,5,6-tetramethylpyrazine 6.8g (50mmol) and 150mL water into a 500mL two-necked flask, stir and heat to 60°C, then add potassium permanganate 15.8g ( 100mmol) and 100mL water suspension, TLC monitors the disappearance of the raw materials and stops the reaction; cool to room temperature, filter with suction, wash the filter cake with water, adjust the pH value of the filtrate to 1-2 with 3N hydrochloric acid, extract with chloroform, combine the organic phases, and anhydrous Dry over sodium sulfate overnight, filter, concentrate to dryness under reduced pressure, and dry in vacuo to obtain 4.32 g of off-white solid with a yield of 52%. 1 H NMR (D 2 O,300MHz)2.55(s,6H,CH 3 ),2.61(s,3H,CH 3 )
Embodiment 3
[0072] Embodiment 3: the synthesis of D001 (intermediate product A)
[0073]
[0074] Dissolve 1.0 g (5.0 mmol) of Danshensu in 15 mL of DMF, and add 1.15 g (6.0 mmol) of EDC·HCl and 0.8 g (6.0 mmol) of 1-hydroxy-benzo-triazole (HOBt) to it, at room temperature Stir evenly under the hood, reduce the temperature of the reaction system to 0° C. with an ice-water bath, add 0.83 g (5.5 mmol) of 2-aminomethyl-3,5,6-trimethylpyrazine prepared in Example 1 dropwise, and The reaction was carried out for 18 hours, and the reaction was monitored by TLC. After the reaction of the raw materials was completed, icy brine (8wt% NaCl, -4°C, the same below) and ethyl acetate were added to the reaction system to dilute, extracted 3 times with ethyl acetate, and ethyl acetate was combined. The ester layer was washed 3 times with ice brine, and the DMF in the organic phase was washed away, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, concentrate under reduced pressure, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com