Method for building N-glycosylation efficiency detection receptor protein models in Escherichia coli by aid of skeleton proteins Fn3 (fibronectin type III domain)
A technology of Escherichia coli and skeleton protein, applied in microorganism-based methods, chemical instruments and methods, biochemical equipment and methods, etc., can solve problems such as inferior flexibility, improve physical and chemical properties, clarify structure-activity relationship, and achieve high efficiency and low cost. The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
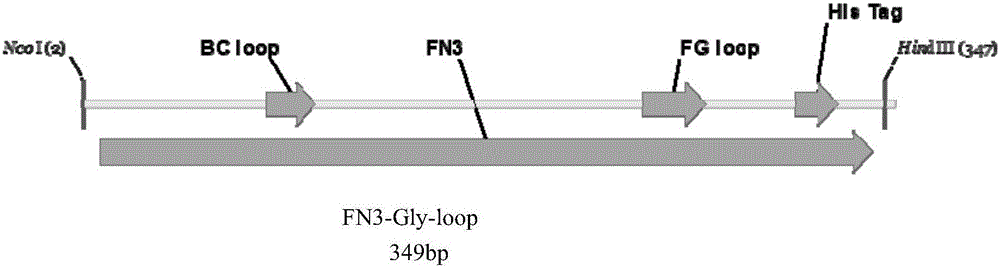
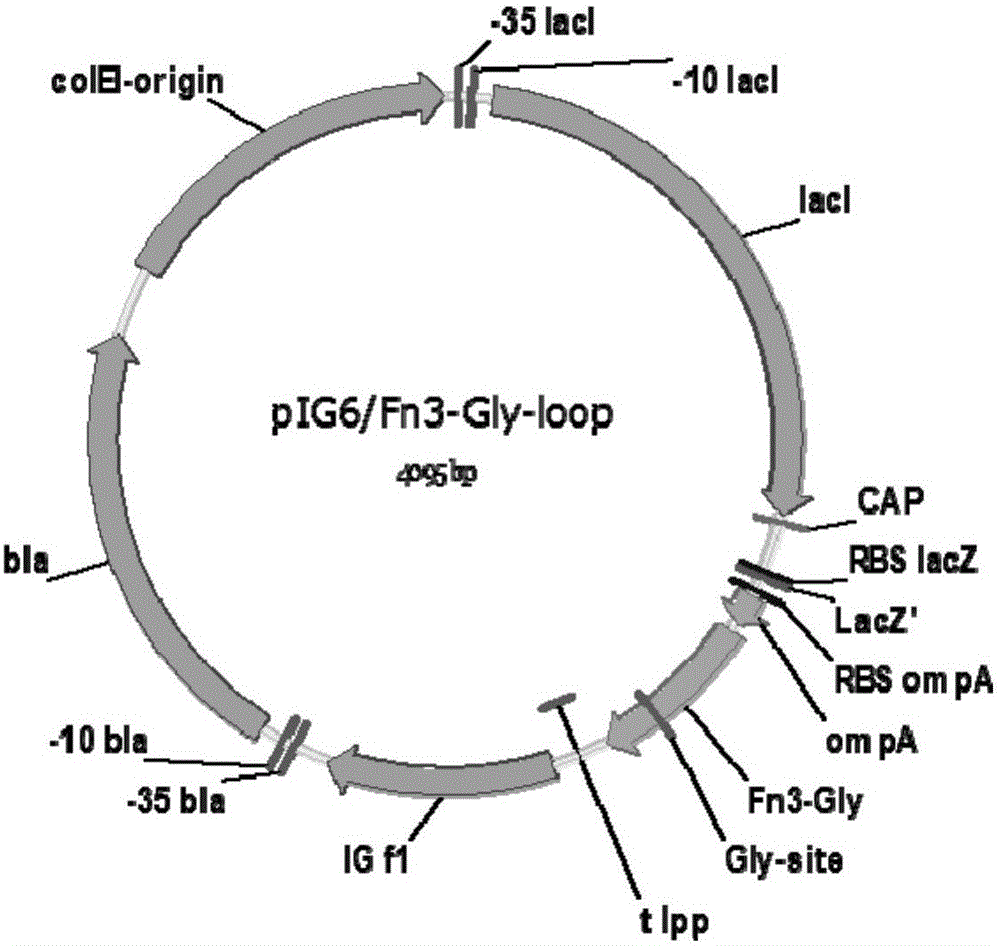
[0018] 1. In this example, human fibronectin type III domain (Fn3) protein was used as a model gene (EMBL accession number AJ320527). At the 5′ end of the gene, 6 histidine residues are introduced, and at the FGloop region, a base sequence encoding the glycosylation site DQNAT is introduced. See the structure of the fusion gene. figure 1 . The gene was constructed into pIG6H by gene recombination, see the vector structure figure 2 .
[0019] 2. After the human fibronectin type III domain mutant Fn3-Gly-loop gene expression cassette sequence shown in the sequence table was synthesized (Nanjing GenScript Biotechnology Co., Ltd.), EcoRV and HindIII were used to construct an E. coli expression vector On pIG6H, the recombinant vector pIG6H-Fn3-Gly-loop was obtained.
[0020] 3. the expression vector constructed and pACYCpgl electric shock transformation CLM37 escherichia coli bacterial strain, then transformants were inoculated to the LB solid medium (per liter of culture) cont...
Embodiment 2
[0024] 1. In this example, human fibronectin type III domain (Fn3) protein was used as a model gene (EMBL accession number AJ320527). At the 5′ end of the gene, 6 histidine residues are introduced, and at the FGloop region, a base sequence encoding the glycosylation site DQNAT is introduced. See the structure of the fusion gene. figure 1 . The gene was constructed into pIG6H by gene recombination, see the vector structure figure 2 .
[0025] 2. After the human fibronectin type III domain mutant Fn3-Gly-loop gene expression cassette sequence shown in the sequence table was synthesized (Nanjing GenScript Biotechnology Co., Ltd.), EcoRV and HindIII were used to construct an E. coli expression vector On pIG6H, the recombinant vector pIG6H-Fn3-Gly-loop was obtained.
[0026] 3. the expression vector constructed and pACYCpgl electric shock transformation CLM37 escherichia coli bacterial strain, then transformants were inoculated to the LB solid medium (per liter of culture) cont...
Embodiment 3
[0030] 1. In this example, human fibronectin type III domain (Fn3) protein was used as a model gene (EMBL accession number AJ320527). At the 5′ end of the gene, 6 histidine residues are introduced, and at the FGloop region, a base sequence encoding the glycosylation site DQNAT is introduced. See the structure of the fusion gene. figure 1 . The gene was constructed into pIG6H by gene recombination, see the vector structure figure 2 .
[0031] 2. After the human fibronectin type III domain mutant Fn3-Gly-loop gene expression cassette sequence shown in the sequence table was synthesized (Nanjing GenScript Biotechnology Co., Ltd.), EcoRV and HindIII were used to construct an E. coli expression vector On pIG6H, the recombinant vector pIG6H-Fn3-Gly-loop was obtained.
[0032] 3. the expression vector constructed and pACYCpgl electric shock transformation CLM37 escherichia coli bacterial strain, then transformants were inoculated to the LB solid medium (per liter of culture) cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com