Targeted prodrug for treating castration-resistant prostate cancer as well as nano preparation and preparation method of targeted prodrug
A technology for castration resistance and prostate cancer, which is applied in the direction of antineoplastic drugs, pharmaceutical formulas, medical preparations of non-active ingredients, etc., can solve the problems of poor water solubility of CPT, and achieve the advantages of simple preparation method and excellent specific recognition effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
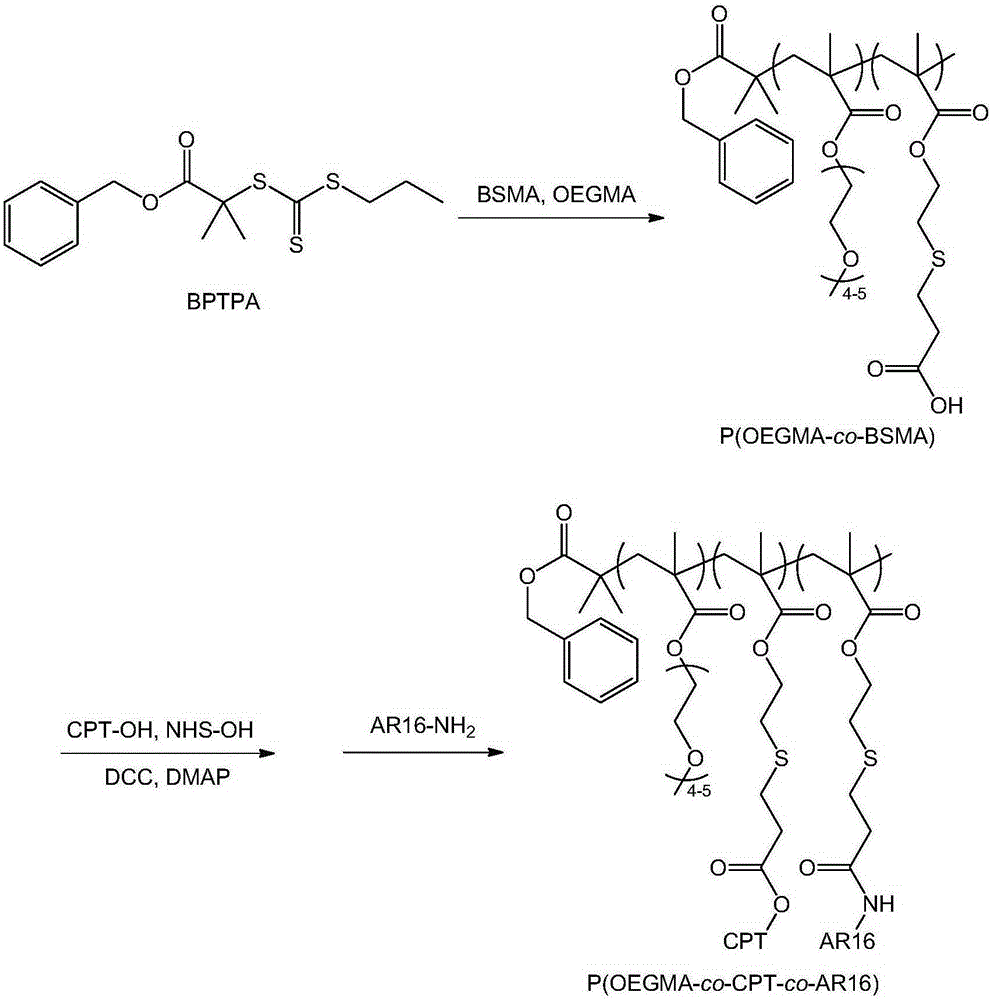
[0045] In a second aspect, the present invention provides a method for preparing the above-mentioned targeted prodrug for treating castration-resistant prostate cancer, the synthetic route of the method is as follows: figure 1 As shown, it specifically includes the following steps:
[0046] (1) Preparation of intermediate product P(OEGMA-co-BSMA):
[0047] Add a certain amount of reversible addition-fragmentation chain transfer initiator trithioester BPTPA, oligoethylene glycol methacrylate OEGMA, monomer BSMA containing β-thiocarboxyl group, solvent and free radical initiator into the glass tube, Freeze and thaw three times in vacuum and seal the tube; then, react at 20-80°C for 0.5-40 hours to obtain the crude product P(OEGMA-co-BSMA); finally purify the crude product P(OEGMA-co-BSMA) Obtain the P(OEGMA-co-BSMA);
[0048] (2) Preparation of target product P(OEGMA-co-CPT-co-AR16):
[0049] Dissolve a certain amount of P(OEGMA-co-BSMA), N-hydroxysuccinimide, CPT, condensing...
Embodiment 1
[0071] AR16 peptide-modified poly(oligoethylene glycol methacrylate-co-camptothecin-co-AR16) macromolecular prodrug specifically targeting castration-resistant prostate cancer, namely P(OEGMA-co- CPT-co-AR16) preparation of targeted prodrug:
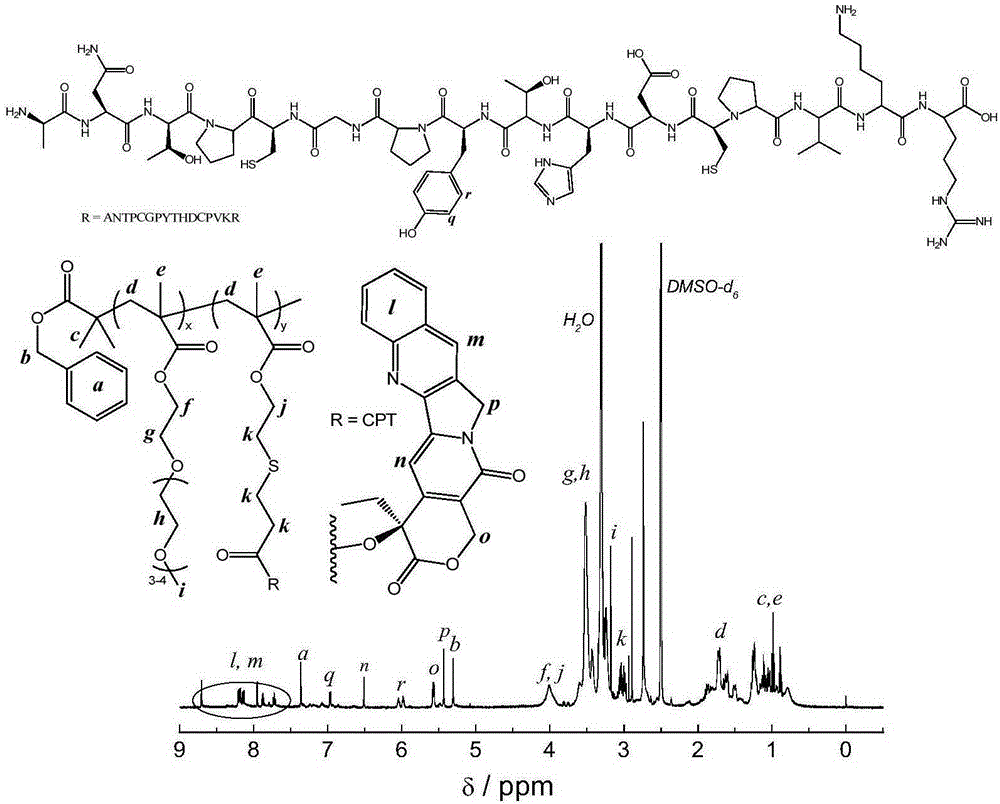
[0072] (1) Preparation of P(OEGMA-co-BSMA): A certain amount of BPTPA (33mg, 0.1mmol), OEGMA (2.70g, 9.0mmol), BSMA (218mg, 1.0mmol) and AIBN (1.6mg, 0.01mmol) Add to glass tube. Add 10 mL of dioxane, vacuum freeze-thaw three times, and then seal the tube. Thereafter, the reaction was carried out at 70° C. for 5 hours. The obtained reaction crude product was dialyzed in water for 24 hours to remove impurities, and freeze-dried. through 1 According to HNMR analysis, the degree of polymerization of P(OEGMA-co-BSMA) was 74, and the molar percentages of OEGMA and BSMA were 88% and 12%, respectively. Determined by GPC, P(OEGMA-co-BSMA) molecular weight M n =22,000, molecular weight distribution M w / M n = 1.02.
[0073] (2) Preparati...
Embodiment 2
[0075] Preparation of P(OEGMA-co-CPT-co-AR16) nano drug solution:
[0076] Dissolve 10 mg of P (OEGMA-co-CPT-co-AR16) in 1 mL of DMSO, drop the solution into 9 mL of stirred water, then put the solution into a dialysis bag (molecular weight cut-off of 7,000 Da) and dialyze for 24 hours to remove the organic solvent. That is, the nano drug solution is obtained. Its particle size is about 85.8nm (±1.56) (see Figure 4 ), potential -4.5mV (±0.18).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Mwco | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com