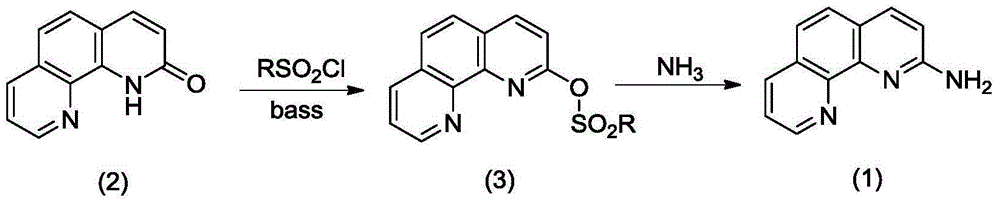
Synthetic method for 2-amino-1,10-phenanthroline
A technology of o-phenanthroline and its synthesis method, which is applied in the field of preparation of 2-amino-1,10-phenanthroline, and can solve the problems of less access, less performance and application research, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] Add 20mL toluene, 1.96g (10mmol) 1,10-phenanthrolin-2-one, 9.53g (50mmol) p-toluenesulfonyl chloride, 5g (50mmol) 1-methylpiperidine in the flask, stir, Heat to 112°C and reflux for 12 hours (TLC tracking), cool slightly, add 20 mL of concentrated ammonia water, heat and reflux for 4 hours, cool, add 20 mL of water, separate layers, extract the aqueous layer with 20 mL of chloroform, combine the organic layers, and concentrate to dryness. The residue was purified by silica gel chromatography (the mobile phase was ethyl acetate) to obtain 1.53 g of white solid, yield 78%, Mp 187-189°C.
Embodiment 2
[0011] Add 20mL xylene, 1.96g (10mmol) 1,10-phenanthrolin-2-one, 11.4g (100mmol) methanesulfonyl chloride, 10.1g (100mmol) triethylamine into the flask, stir and heat to 70°C React for 6h (TLC tracking), cool slightly, add 20mL of concentrated ammonia water, heat to reflux for 6h, cool, add 40mL of water, separate layers, extract the aqueous layer with 20mL of chloroform, combine the organic layers, concentrate to dryness, and wash the residue with silica gel layer Purification by column analysis (mobile phase is ethyl acetate) to obtain 0.22 g of white solid, yield 11%, Mp 187-189°C.
Embodiment 3
[0013] Add 20mL dimethyl sulfoxide, 1.96g (10mmol) 1,10-phenanthrolin-2-one, 9.53g (50mmol) benzylsulfonyl chloride, 5.4g (50mmol) sodium carbonate in the flask, stir and heat React at 90°C for 6h (TLC tracking), cool slightly, add 20mL of concentrated ammonia water, heat to reflux for 4h, concentrate the solvent under reduced pressure, add 20mL of water to the residue, extract the aqueous layer with 20mL*2 chloroform, concentrate the extract to dryness, and leave The compound was purified by silica gel column chromatography (mobile phase was ethyl acetate) to obtain 1.0 g of white solid, yield 51%, Mp 187~189°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com