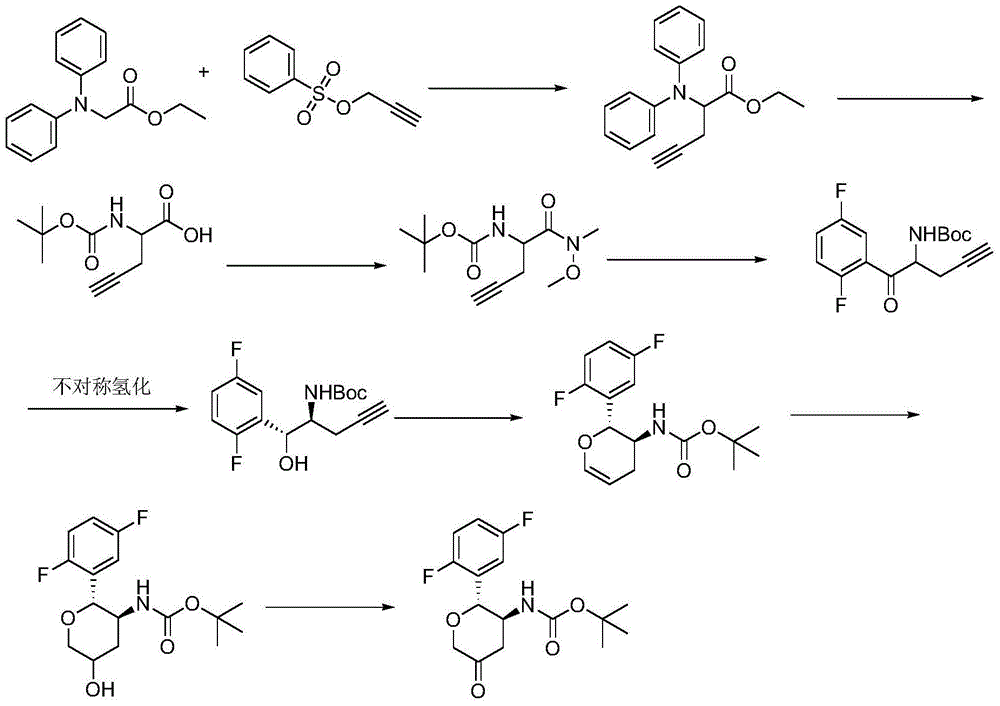
A kind of preparation method of compound
A technology for compound and chemical separation, applied in the direction of organic chemistry, etc., can solve the problems of difficult control of reaction conditions, unfavorable industrial production, long synthesis route, etc., and achieves the effect of omitting the asymmetric hydrogenation reaction step, low price, and improving yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
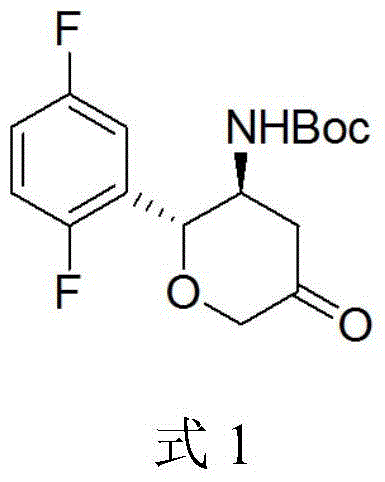
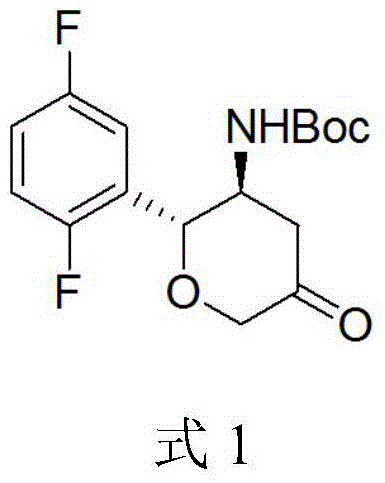
[0021] 1,1-Dimethylethyl N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]-carbamate preparation method
[0022] Take 10 g of compound 3 and 11 g of D-tartaric acid and add it into 100 ml of methanol, heat to reflux, then cool to room temperature and stir for 4 hours, and filter to obtain the D-tartrate salt of compound 3. Add the D-tartrate of compound 3 into 50ml of water, stir to dissolve, then add sodium hydroxide to adjust the pH to 9, then add 5g of di-tert-butyl dicarbonate for reaction, add ethyl acetate to extract after the reaction, concentrate to dryness to obtain white solid. Add 20ml of acetonitrile, 10ml of glacial acetic acid, 0.04ml of ruthenium trichloride and 10ml of aqueous solution to the white solid, then add 1.7g of sodium bromate, the reaction is complete at 3°C, then add 100ml of water, filter the precipitated solid, and water the solid Washing gave 5.4 g of compound 1 as a white solid with an optical purity of 99.8% e.e.
Embodiment 2
[0024] 1,1-Dimethylethyl N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]-carbamate preparation method
[0025] Take 10 g of compound 3 and 11 g of D-tartaric acid and add it into 150 ml of ethanol, heat to reflux, then cool to room temperature and stir for 4 hours, and filter to obtain the D-tartrate salt of compound 3. Add the D-tartrate of compound 3 into 50ml of water, stir to dissolve, then add sodium hydroxide to adjust the pH to 8, then add 3g of di-tert-butyl dicarbonate, after the reaction is complete, add ethyl acetate for extraction, and concentrate to dryness to obtain a white solid . Add 20ml of acetonitrile, 10ml of glacial acetic acid, 0.04ml of ruthenium trichloride and 10ml of aqueous solution to the white solid, then add 1.7g of sodium bromate, the reaction is complete at 0-5°C, then add 100ml of water, filter the precipitated solid, The solid was washed with water to obtain 6 g of white solid compound 1 with an optical purity of 99.0% e.e. ...
Embodiment 3
[0027] 1,1-Dimethylethyl N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]-carbamate preparation method
[0028] Take 10g of compound 3 and 11g of D-tartaric acid and add it to a mixed solution of 80ml of methanol and 40ml of ethanol, heat to reflux, then cool to room temperature and stir for 4 hours, and filter to obtain the D-tartrate of compound 3. Add the D-tartrate of compound 3 into 50ml of water, stir to dissolve, then add sodium hydroxide to adjust the pH to 8-9, then add 5g of di-tert-butyl dicarbonate, after the reaction is complete, add ethyl acetate to extract, and concentrate to dryness to obtain white solid. Add 20ml of acetonitrile, 10ml of glacial acetic acid, 0.04ml of ruthenium trichloride and 10ml of aqueous solution to the white solid, then add 1.7g of sodium bromate, the reaction is complete at 0-5°C, then add 100ml of water, filter the precipitated solid, The solid was washed with water to obtain 6 g of white solid compound 1 with an opti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com