Copper-fine-particle dispersion liquid, conductive-film formation method, and circuit board
A technology of copper particles and dispersions, applied to the formation of conductive patterns, conductive materials dispersed in non-conductive inorganic materials, conductive layers on insulating carriers, etc., can solve problems such as low resistance, failure to form, and insufficient photo-sintering , to achieve the effect of low resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
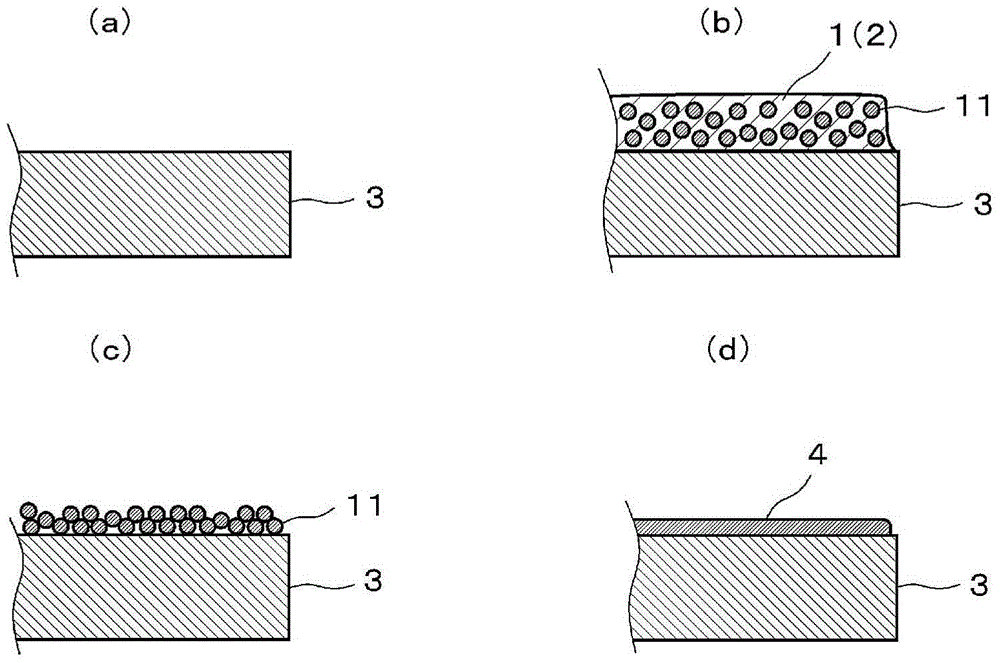
[0045] A copper particle dispersion is prepared in which a dispersant is added to disperse the copper particles in a dispersion medium. The dispersion medium is alcohol (diethylene glycol). This dispersion medium is also used as a sintering aid in this embodiment. The dispersant was phosphoric acid ester (trade name: "DISPERBYK (registered trademark)-102", available from BYK-Chemie). The concentration of the dispersant was 2% by mass relative to the copper fine particle dispersion. The copper fine particles used had an average particle size of 50 nm, and the concentration of the copper fine particles was 40% by mass. Glass slides were used as substrates.
[0046] The copper particle dispersion was applied to the substrate by spin coating to form a coating with a thickness of 1 μm. Coating color is black. The coating is irradiated with light without drying. Light irradiation energy is 14J / cm 2 .
[0047] The appearance of the coating changes to that of metallic copper b...
Embodiment 2
[0049] The dispersion medium used was an alcohol (diethylene glycol monoethyl ether) different from Example 1. This dispersion medium is also used as a sintering aid in this embodiment. Unlike Example 1, the dispersant used was a phosphoric acid ester (trade name: "DISPERBYK (registered trademark)-111", available from BYK-Chemie). Other conditions are identical with embodiment 1. The appearance of the coating changes to that of metallic copper by light irradiation, and a conductive film is formed on the substrate. The sheet resistance of the formed conductive film was 500 mΩ / sq.
Embodiment 3
[0051] Copper particle dispersions were prepared using copper particles having an average particle size of 70 nm. Then, phosphoric acid ester (trade name: "DISPERBYK (registered trademark)-111", available from BYK-Chemie) was added as a sintering aid to the copper particle dispersion. The sintering aid also acts as a dispersant. The concentration of the sintering aid was 10% by mass relative to the copper fine particle dispersion. A glass substrate (trade name "EAGLEXG (registered trademark)", available from Corning) was used as the substrate. Other conditions are identical with embodiment 2. A coating comprising a dispersion of copper particles is formed on a substrate. After the coating dries, the coating is irradiated with light. The energy of light irradiation is 11J / cm 2 . The appearance of the coating changes to that of metallic copper by light irradiation, and a conductive film is formed on the substrate. The sheet resistance of the formed conductive film was 170...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com