Cryopreservation solution for tendon stem cells from achilles tendon of human and preparation method of cryopreservation solution
A tendon stem cell and cryopreservation technology, applied in the field of stem cells, can solve problems such as being unsuitable for clinical application, affecting treatment results, contaminating allergens, etc., to avoid the introduction of heterologous substances, high clinical safety, and cell damage. small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
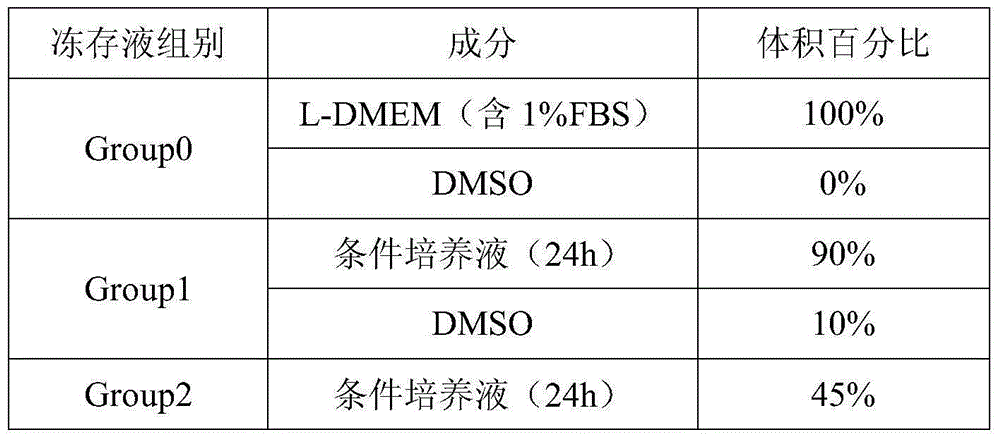
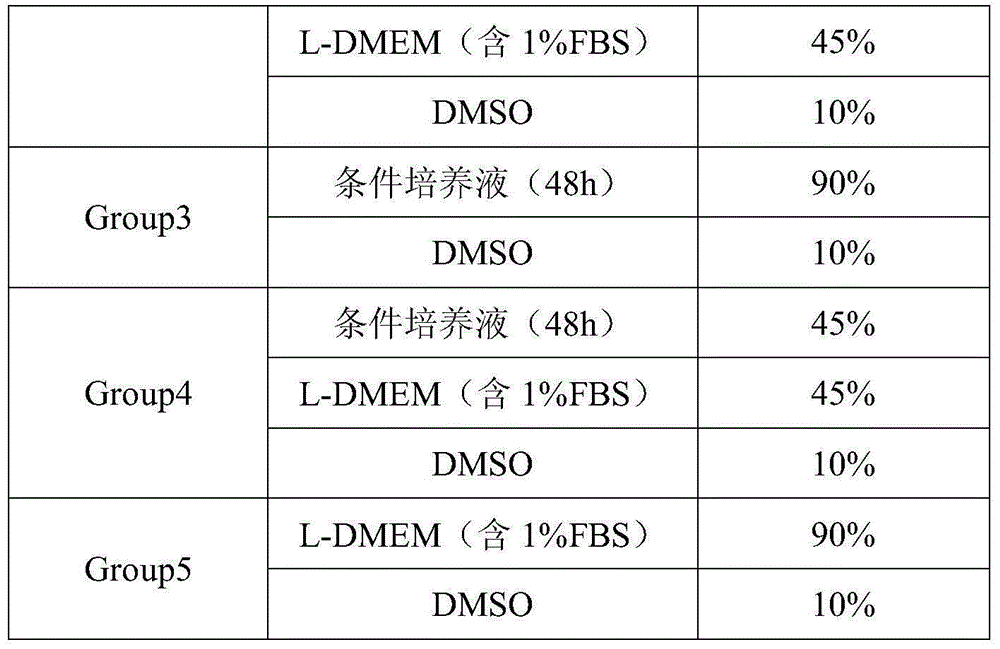
[0023] Embodiment 1, human Achilles tendon-derived tendon stem cell cryopreservation solution
[0024] Tendon stem cells were cultured in L-DMEM containing 10% FBS at 37°C, 5% CO 2 Cultivate under the condition of 2-3 days, subculture at 1:5; take the tendon stem cells in the logarithmic growth phase, make cell suspension with cell culture medium, adjust the cell concentration to 1×10 5 cell / mL, take 15mL of cell suspension and inoculate it on a 10cm-diameter petri dish, at 37°C, 5% CO 2 The cell culture medium is L-DMEM containing 1% FBS.
[0025] When culturing to the 24th hour, collect the supernatant for later use, and replace the new cell culture medium to continue culturing; when cultivating to the 48th hour, collect the supernatant again, and centrifuge the supernatant collected twice at 2000RPM for 5min, Remove cell debris and store at a constant temperature of 4°C for later use. Prepare cryopreservation solution for cryopreserved cells and conventional cell cryopre...
Embodiment 2
[0029] Example 2. Cryopreservation, resuscitation and viability of tendon stem cells derived from human Achilles tendon
[0030] The final density of cryopreserved tendon stem cells derived from human Achilles tendon is 1-5×10 6 cell / mL, cryopreservation volume is 1.5mL / tube, cryopreservation according to the following procedures: first place at 4°C for 2h, then place in liquid nitrogen port for 30min, and finally put into liquid nitrogen (-196°C).
[0031] After 2 weeks of cryopreservation, the cells were resuscitated. Each group resuscitated 3 tubes. The cryopreservation tubes were taken out of the liquid nitrogen and placed directly in warm water at 37°C, and shaken from time to time to melt as soon as possible in a short time. Centrifuge in a centrifuge for 5 minutes, discard the supernatant, add 1mL cell culture medium to each tube, count and calculate the average value. Specifically, the assay methods for cell count and cell viability are as follows: 1. trypsinize the a...
Embodiment 3
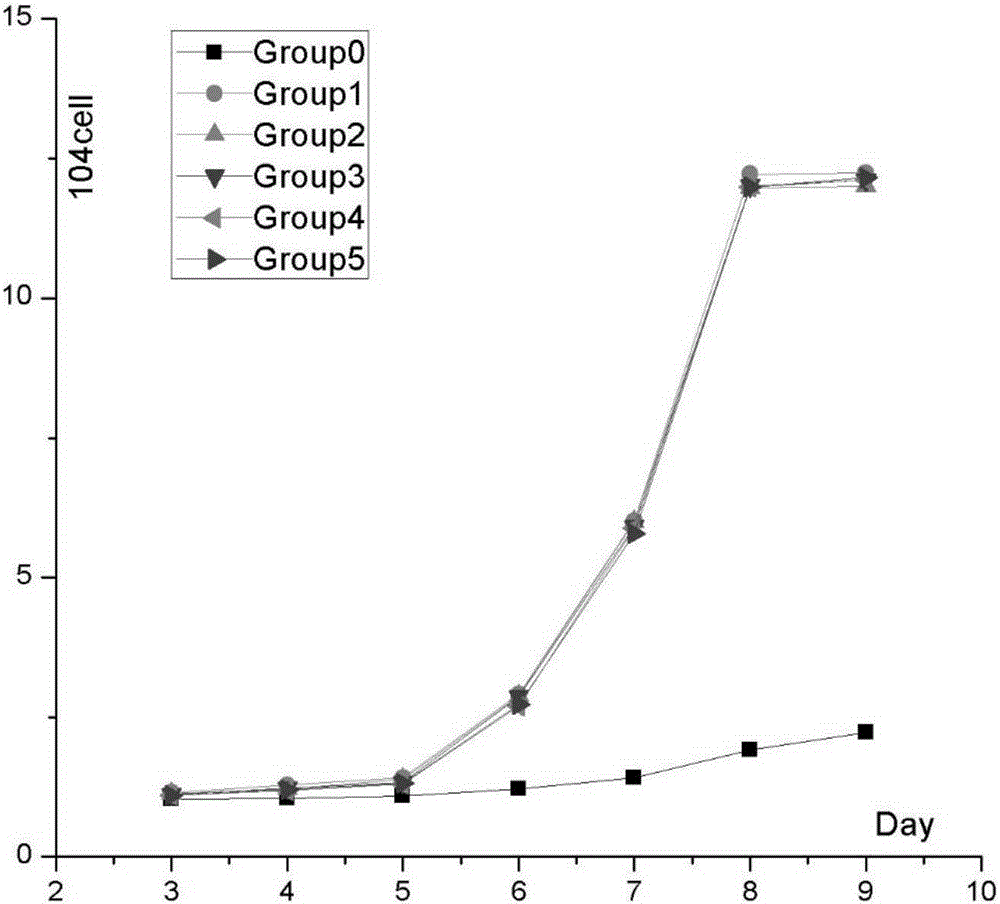
[0035] Example 3, Proliferation Ability of Cells After Recovery
[0036] Take a 12-well culture plate, inoculate each well with the recovered cells at a density of 10,000 cells / well, add 1 mL of L-DMEM solution containing 10% FBS to each well, and place at 37°C, 5% CO 2Cultured in an incubator, the medium was changed every three days. From the 3rd day (the newly recovered cells need about 24 hours to adapt to the environment), take out the plate every 24 hours, randomly select 3 wells, aspirate the old culture medium and wash it with PBS, add trypsin to digest the cells, stop the digestion, and prepare single cells Suspension, blow evenly, take 10 μL of cell suspension and 10 μL of 0.4% trypan blue, mix well, add the sample to the blood cell counting plate, count the number of cells in the large squares at the four corners of the counting plate under a 10-fold microscope, and count the number of cells As shown in Table 3, the cell growth curve is drawn with time as the horizo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com