O-dichlorobenzene synthesizing method
A technology of o-dichlorobenzene and synthesis method, which is applied in the fields of chemical instruments and methods, organic compound/hydride/coordination complex catalyst, organic chemistry, etc. It can solve the problems of many by-products, complicated synthesis process, and low separation efficiency, etc. problems, to achieve the effect of reducing by-products, optimizing reaction conditions, and improving economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
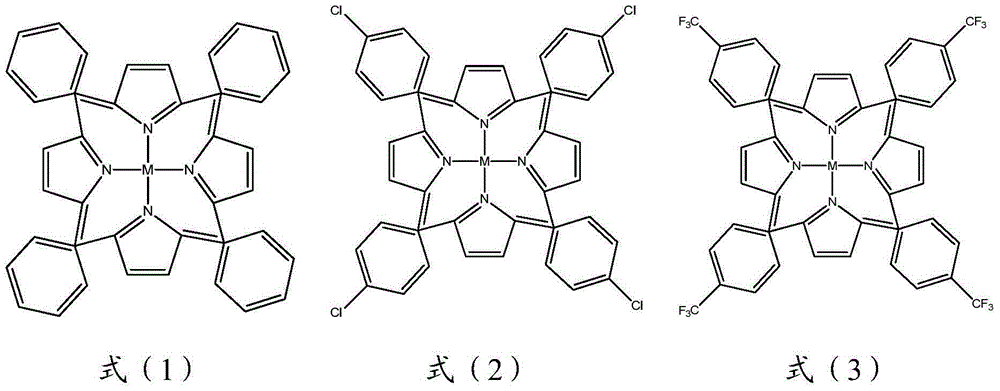
[0023] 78 kilograms of benzene and 0.1 kilogram of catalyzer (5,10,15,20-tetraphenyliron porphyrin, concrete structural formula as shown in the above formula (1), M is iron ion) is added in the reactor, feeds 145 kilograms of chlorine gas Reaction, the reaction temperature is 70-120 degrees, the reaction pressure is 1-4 atmospheres, and the reaction time is 2-7 hours. Absorb the hydrogen chloride gas generated by the reaction with an appropriate amount of water. The reacted solution was distilled to obtain a mixture of chlorobenzene and o-dichlorobenzene. The mixture was washed with a 5% sodium hydroxide solution, dried, and rectified to obtain 135 kg of o-dichlorobenzene with a purity of 99.5%. and a small amount of chlorobenzene. The residue in the reactor was directly used for subsequent reactions.
[0024] The yield of o-dichlorobenzene of this embodiment is 98%.
Embodiment 2
[0026] The residue after distillation in 78 kilograms of benzene and embodiment 1 (main component is 5,10,15,20-tetraphenyliron porphyrin) is added in reaction unit, feeds 145 kilograms of chlorines and reacts, and temperature of reaction is 70 -120 degrees, the reaction pressure is 1-4 atmospheres, and the reaction time is 2-7 hours. Absorb the hydrogen chloride gas generated by the reaction with an appropriate amount of water. The reacted solution was distilled to obtain a mixture of chlorobenzene and o-dichlorobenzene. The mixture was washed with a 5% sodium hydroxide solution, dried, and rectified to obtain 132 kg of o-dichlorobenzene with a purity of 99.5%. and a small amount of chlorobenzene. The residue in the reactor was directly used for subsequent reactions.
[0027] The yield of o-dichlorobenzene in this embodiment is 95.8%.
Embodiment 3
[0029] The residue (main component is 5,10,15,20-tetraphenyliron porphyrin) after the distillation in 78 kilograms of benzene and embodiment 2 is added in the reactor, feeds 145 kilograms of chlorines and reacts, and reaction temperature is 70 -120 degrees, the reaction pressure is 1-4 atmospheres, and the reaction time is 2-7 hours. Absorb the hydrogen chloride gas generated by the reaction with an appropriate amount of water. The reacted solution was distilled to obtain a mixture of chlorobenzene and o-dichlorobenzene. The mixture was washed with 5% sodium hydroxide solution, dried, and rectified to obtain 133 kilograms of o-dichlorobenzene with a purity of 99.5%. A small amount of chlorobenzene. The residue in the reactor was directly used for subsequent reactions.
[0030] The yield of o-dichlorobenzene in this embodiment is 96.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com