Improved propacetamol hydrochloride preparation process
A technology of propetamol hydrochloride and propetamol, which is applied in the field of synthesis of organic compounds with carbocyclic rings, can solve the problems of difficulty in the refining process, high requirements for raw and auxiliary materials, and many times of refining, and achieve operational process Easier to control, reduce product cost, and reduce the effect of many by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
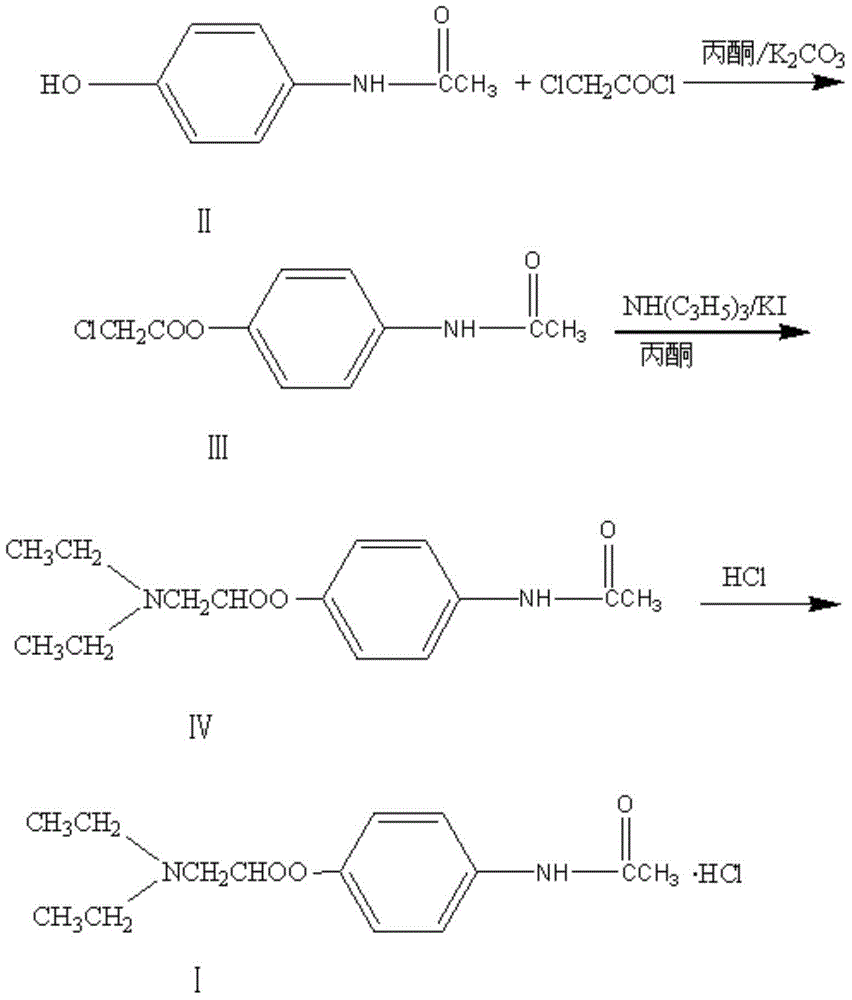
Image
Examples
Embodiment 1
[0028] 1. Add 120kg of acetone and 10kg of acetaminophen to a 200L reactor, stir to dissolve, add 24kg of anhydrous potassium carbonate, stir and cool. Control the temperature of 15-20 DEG C to add 7.5 kg of chloroacetyl chloride dropwise, and complete the dropwise addition in 1.8 hours, and control the temperature for 1.5 hours to complete the reaction. Add 0.5 kg of potassium iodide and control the temperature at 15-20°C and add 11.5 kg of diethylamine dropwise. After the addition of diethylamine in 2 hours, the temperature was controlled to react for 50 minutes and filtered, and the filter cake was washed with acetone. The collected filtrate was concentrated in vacuo to obtain a viscous crude product of Propatamol.
[0029] 2. Add 40 kg of chloroform and 30 kg of purified water to the viscous crude product of propatamol, stir and purify, extract the chloroform layer, and concentrate the chloroform under reduced pressure to obtain the product of propatamol.
[0030] 3. Add 80kg...
Embodiment 2
[0033] 1. Add 120kg of acetone and 10kg of acetaminophen to a 200L reactor, stir to dissolve, add 24kg of anhydrous potassium carbonate, stir and cool. Control the temperature to 20-25°C and add 7.5kg of chloroacetyl chloride dropwise, and the dropwise addition is completed in 1.6 hours, and the temperature is controlled to react for 1.5 hours, and the reaction is completed. Add 0.5 kg of potassium iodide and control the temperature to 20-25°C and add 11.5 kg of diethylamine dropwise. After the addition of diethylamine in 2 hours, the temperature was controlled to react for 50 minutes and filtered, and the filter cake was washed with acetone. The collected filtrate was concentrated in vacuo to obtain a viscous crude product of Propatamol.
[0034] 2. Add 40 kg of chloroform and 30 kg of purified water to the viscous crude product of propatamol, stir and purify, extract the chloroform layer, and concentrate the chloroform under reduced pressure to obtain the product of propatamol...
Embodiment 3
[0038] 1. Add 120kg of acetone and 10kg of acetaminophen to a 200L reactor, stir to dissolve, add 24kg of anhydrous potassium carbonate, stir and cool. Control the temperature at 20-25°C and add 7.5 kg of chloroacetyl chloride dropwise, and finish the dripping in 1.5 hours, and control the temperature to react for 1.5 hours, and the reaction ends. Add 0.5 kg of potassium iodide and control the temperature at 15-20°C and add 11.5 kg of diethylamine dropwise. After the addition of diethylamine in 2 hours, the temperature was controlled to react for 50 minutes and the filter cake was washed with acetone. The collected filtrate was concentrated in vacuo to obtain a viscous crude product of Propatamol.
[0039] 2. Add 40 kg of chloroform and 30 kg of purified water to the viscous crude product of propatamol, stir and purify, extract the chloroform layer, and concentrate the chloroform under reduced pressure to obtain the product of propatamol.
[0040] 3. Add 80kg of absolute ethanol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com