Paclitaxel phospholipid compound and drug combination and application thereof
A technology of phospholipid compounds and paclitaxel analogs, applied in the field of medicine, can solve the problems of slow drug speed, paclitaxel phospholipid prodrugs or liposome drug efficacy decline, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
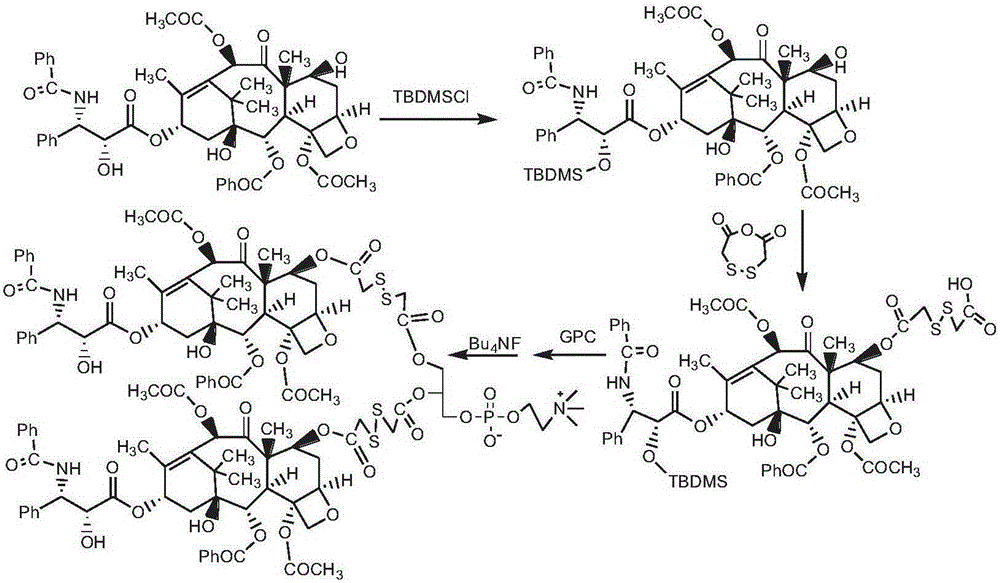
[0117] Synthesis of bis(paclitaxel-7-dithioglycolic acid) phosphatidylcholine (route see figure 1 )
[0118] Dissolve paclitaxel 1.5g (1mmol) in 30mL chloroform, add 1mL triethylamine, add 1.5g tert-butyldimethylsilyl chloride (TBDMSCl), react at 0°C for 3h, add glacial ether, a white precipitate precipitates, centrifuge Separation, further separation by column chromatography (eluent, chloroform / methanol: 7 / 1, v / v) to obtain 2'-tert-butyldimethylsilyloxy-paclitaxel 1.24g , as a white powdery solid.
[0119] 0.20 g of 2'-tert-butyldimethylsilyloxy-paclitaxel was dissolved in 10 ml of chloroform, 0.12 g (1 mmol) of DMAP and 0.6 g of dithiodiethanol anhydride were added, and the reaction was stirred at room temperature for 24 h. Then, wash the above reaction solution with 2% HCl solution, remove DMAP, and separate by column chromatography (eluent, chloroform / methanol: 5 / 1, v / v) to obtain white powder 2'-tert-butyldi Methylsiloxy-paclitaxel-7-dithioglycolic acid monoester 0.13g...
Embodiment 2
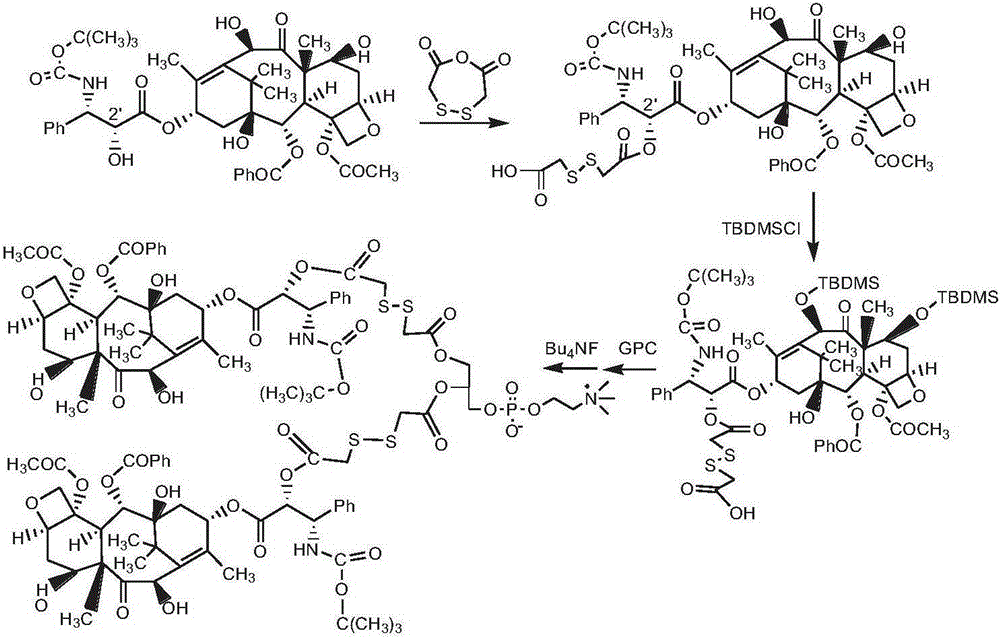
[0122] Synthesis of Bis(docetaxel-2'-dithiodieglycolic acid) phosphatidylcholine (see the synthetic route figure 2 )
[0123] Docetaxel 0.6g was dissolved by adding 30ml of chloroform, DMAP0.3g and dithiodiethanol anhydride 0.64g (6mmol) were added, and the reaction was stirred at room temperature for 24h. Then, wash the above reaction solution with 2% HCl solution, remove DMAP, and separate by column chromatography (eluent, chloroform / methanol: 5 / 1, v / v) to obtain white powdery docetaxel-2'- Dithiodiglycolic acid monoester 0.43g.
[0124] Dissolve 0.20 g of docetaxel-2'-dithiodiethanol monoester in 20 ml of pyridine, add 0.4 g of tert-butyldimethylsilyl chloride, react at room temperature for 3 h, and add the reaction solution dropwise into 50 ml of ice ether, A white precipitate was precipitated, separated by centrifugation, and further separated by column chromatography (eluent, chloroform / methanol: 7 / 1, v / v) to obtain the end-capped product 7,10-bis(tert-butyldimethylsi...
Embodiment 3
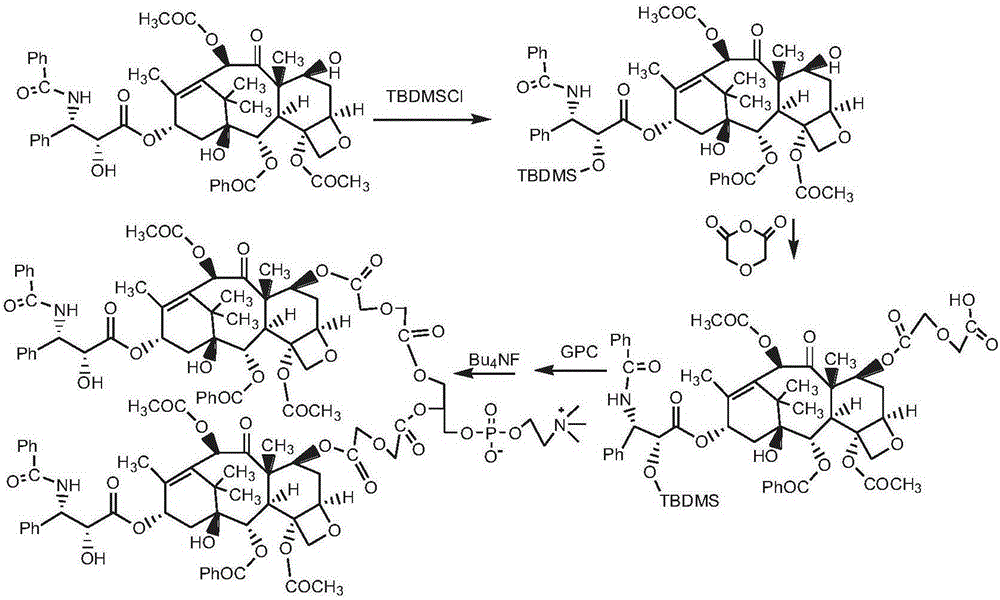
[0128] Synthesis of bis(paclitaxel-7-diglycolic acid) phosphatidylcholine (route see image 3 )
[0129] Dissolve 0.20 g of paclitaxel 2'-blocked product 2'-tert-butyldimethylsiloxy-paclitaxel in Example 1 in 10 ml of chloroform, add 0.5 g of DMAP and 0.5 g of diglycolic anhydride, and stir the reaction at room temperature 24h. Then, the above reaction solution was washed with 2% HCl solution to remove DMAP and separated by column chromatography (eluent, chloroform / methanol: 5 / 1, v / v) to obtain white powder 2'-tert-butyl Dimethylsilyloxy-paclitaxel-7-diglycolic acid monoester. Dissolve 2'-tert-butyldimethylsilyloxy-paclitaxel-7-diglycolic acid monoester in 5 mL of chloroform, add CDI0.1g, activate for 1h, add GPC0.05g and DBU0.1g, react at room temperature for 24h , the reaction solution was treated with 0.5 g of tetrabutylammonium fluoride to remove TBDMS, and the reaction solution was purified by column chromatography to obtain 0.09 g of bis(paclitaxel-7-diglycolic acid) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com