Heavy metal precipitant, preparation method thereof and heavy metal wastewater treatment method
A heavy metal precipitation and wastewater treatment technology, applied in the direction of flocculation/sedimentation water/sewage treatment, water/sewage treatment, water/sludge/sewage treatment, etc., can solve the problems of difficult control and high cost, and achieve simple control and reduce The effect of preparation cost and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
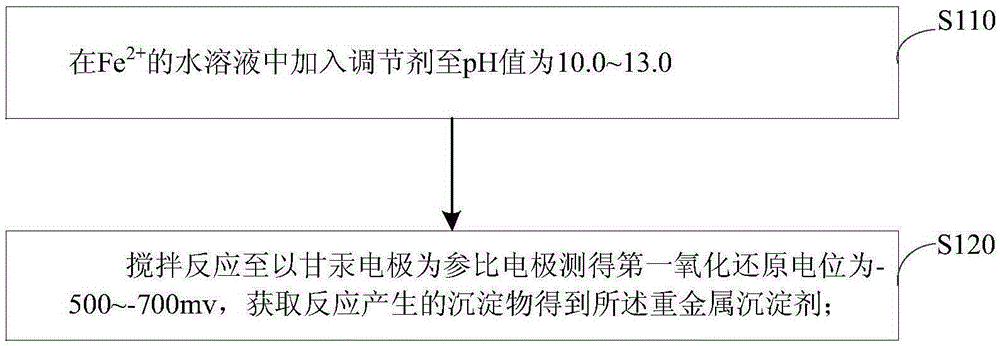
[0030] refer to figure 1 , the preferred embodiment of the present invention provides a kind of preparation method of heavy metal precipitation agent, comprises the following steps:
[0031] S110: in Fe 2+ A regulator is added to the aqueous solution until the pH value is 10.0-13.0.
[0032] S120: Stir the reaction at 10-35° C. until the first oxidation-reduction potential measured with the calomel electrode as the reference electrode is -500--700 mv, and obtain the precipitate produced by the reaction to obtain a heavy metal precipitant.
[0033] Among them, the regulator is NaOH, KOH, Ca(OH) 2 one or more of.
[0034] Fe 2+ The aqueous solution can be prepared by adding ferrous salts to water, and the concentration of ferrous salts is limited to the fact that the ferrous salts added are insoluble in water, that is, Fe 2+ The aqueous solution is a saturated solution. The regulator is an alkaline substance, and the regulator is NaOH, KOH, Ca(OH) 2 one or more of. The a...
Embodiment 1
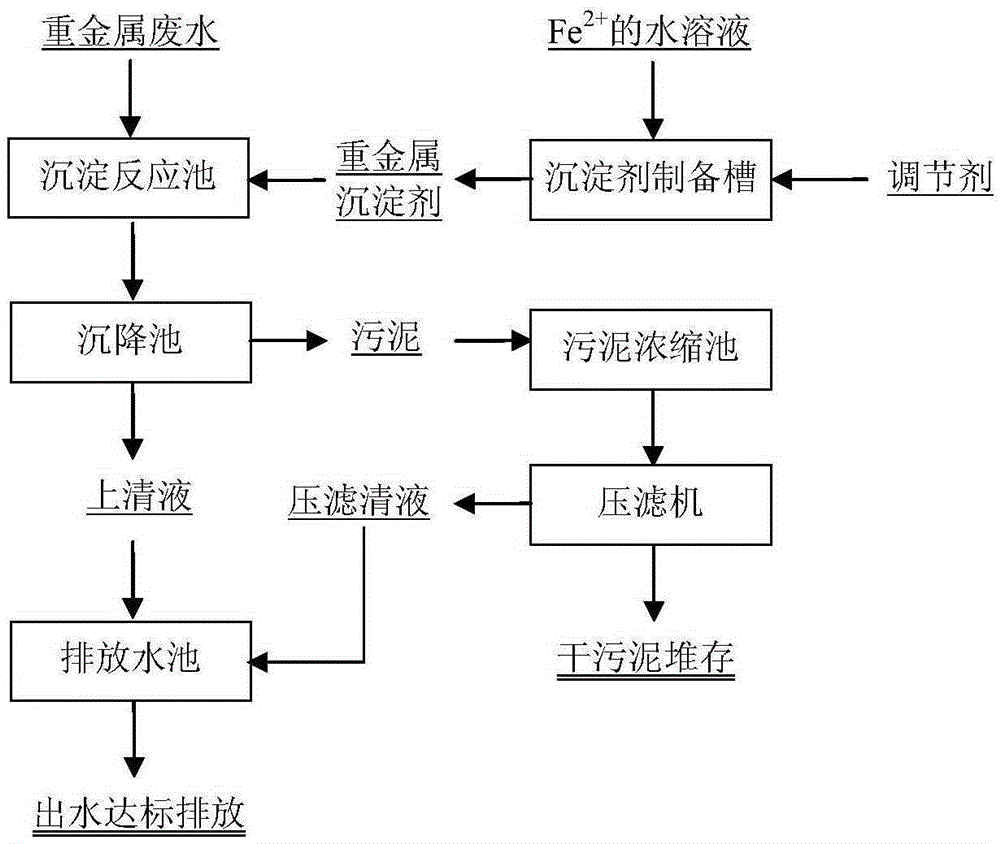
[0053] refer to image 3 Preparation of heavy metal precipitant and treatment of heavy metal wastewater.
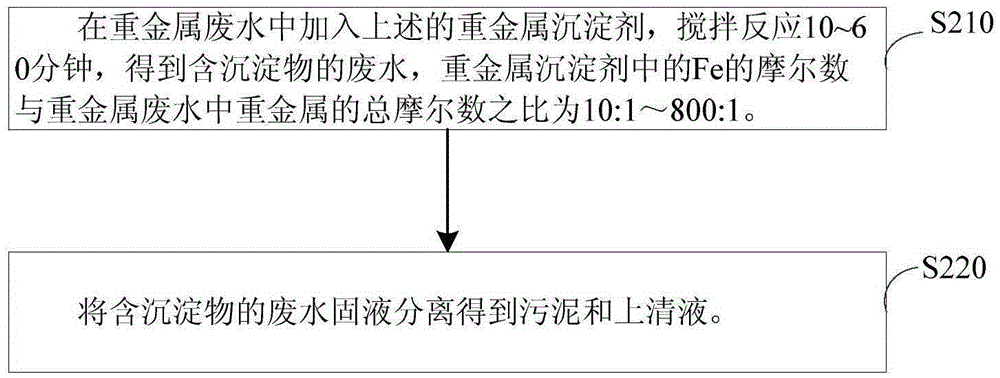
[0054] Add 30L of tap water and 0.3kg of ferrous sulfate (heptahydrate) to the heavy metal precipitant preparation tank, turn on the agitator, continue to add sodium hydroxide to the preparation tank, adjust the pH of the solution to 10.0, and the solution temperature to 20°C. When the oxidation-reduction potential of the solution in the preparation tank reaches -600mv, stop stirring, and the precipitation in the preparation tank is the heavy metal precipitant (1#). After determination, the precipitant quality (dry weight) is 0.056kg, and Fe in the precipitant 3+ with Fe 2+ The molar ratio of = 2:1.
[0055] Heavy metal wastewater (1m 3 ) enters the sedimentation reaction tank after the wastewater regulating tank, and simultaneously adds the prepared heavy metal precipitant (1#) in the wastewater, and stirs and reacts for 0.5h. The ratio of the numbers is 11.3:1, the...
Embodiment 2
[0059] refer to image 3 Preparation of heavy metal precipitant and treatment of heavy metal wastewater.
[0060] The difference from Example 1 is that in Example 2, the ferrous salt is 20.0 kg of ferrous sulfate (heptahydrate), KOH is added to the preparation tank, the pH of the solution is adjusted to 11.0, and the solution temperature is 10°C. When the redox potential of the solution in the preparation tank reaches -500mv, stop stirring. After measuring, the precipitant quality (dry weight) in the preparation tank is 3.733kg, and Fe in the precipitant 3+ with Fe 2+ The molar ratio of =2:1.5. When processing heavy metal wastewater, the stirring reaction time in the precipitation reaction tank is 10 minutes. At this time, the ratio of the molar number of Fe in the heavy metal precipitant to the total molar number of heavy metals in the wastewater is 753.3:1, and the pH value is 8.0. The second The oxidation-reduction potential is -100mv.
[0061] After being treated by t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com