Synthesis method of cardanol-based epoxy resin
A cardanol-based epoxy resin and a synthetic method technology, applied in the field of synthesis of cardanol-based epoxy resin, can solve the problems of difficult control of reaction conditions, increased preparation cost, and difficult product separation, and achieve convenient preparation and epoxy resin Effect with high value and simple response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] step one
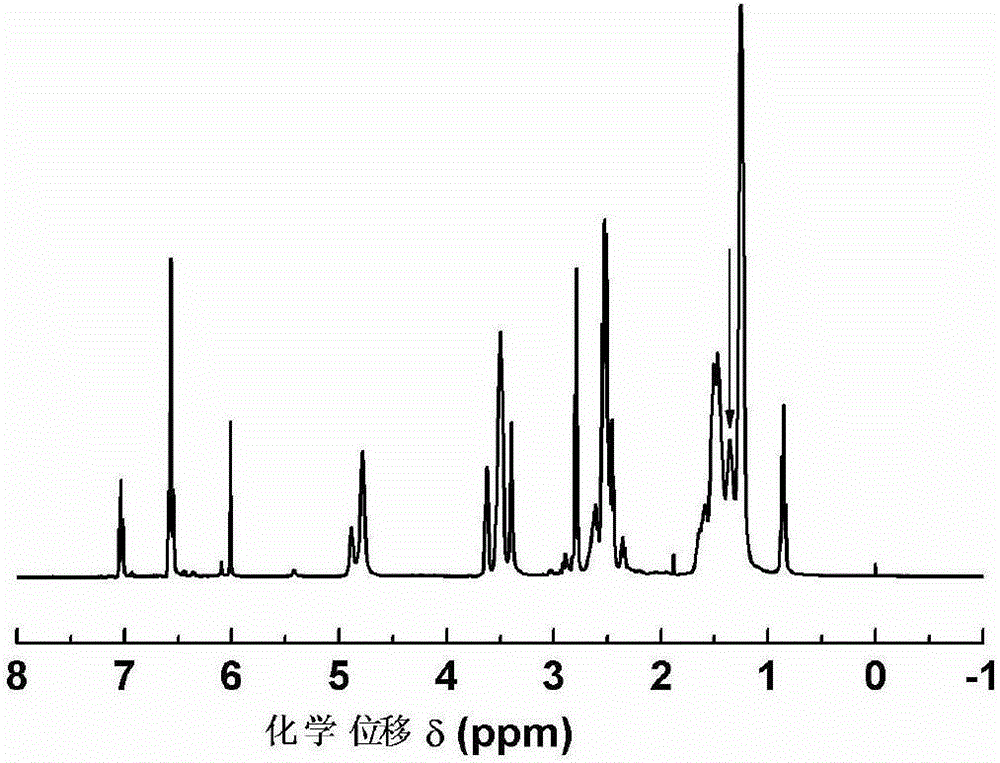
[0025] The side chain double bond of cardanol-thiol click chemical reaction: put 3.00g of cardanol and 0.86g of mercaptoethanol in a quartz flask, blow nitrogen, irradiate with ultraviolet light for 6 hours, and distill under reduced pressure at 100°C to get cashew nuts Hydroxylation product I of phenolic side chain double bond, such as figure 1 shown;
[0026] step two
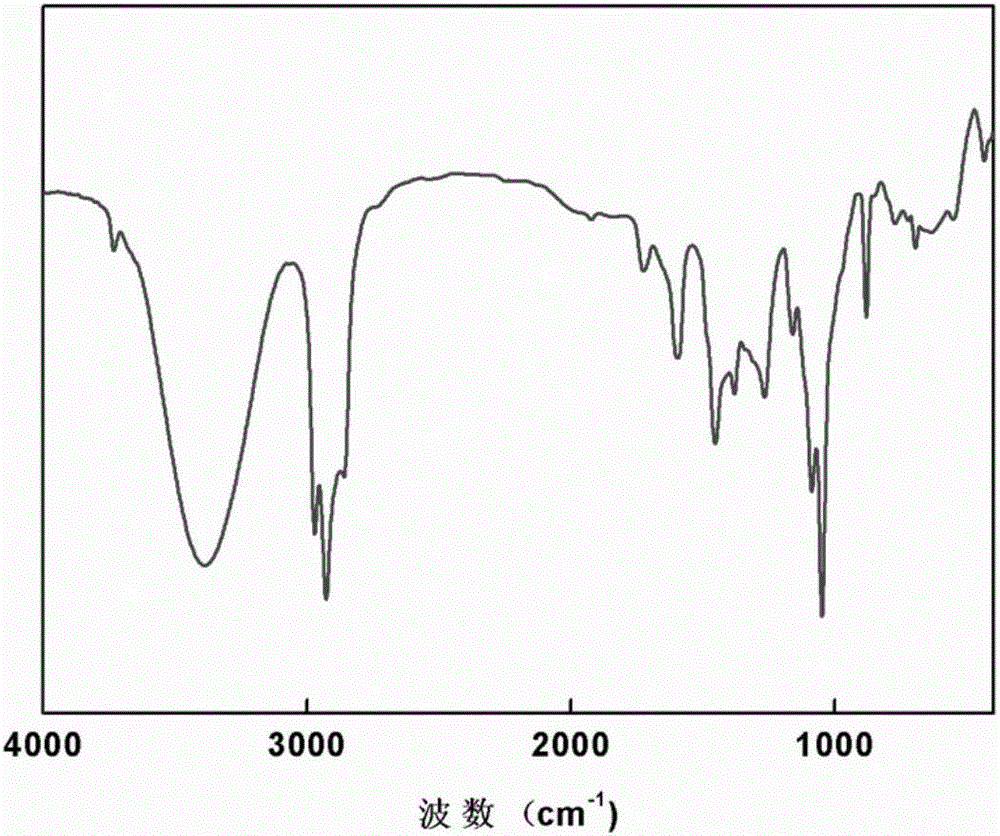
[0027] Epoxidation reaction: 3.78g cardanol side chain double bond hydroxylation product I was added together with 4.15g potassium carbonate and 52.94g dioxane into a 100mL three-necked flask, and the temperature was raised to 60°C with nitrogen gas. Stir at a speed of 1 / min, add dropwise 2.13g epichlorohydrin in 10 minutes, keep warm for 2 hours, filter, and the filtrate obtains cardanol-based epoxy resin through decompression distillation, such as figure 2 Shown, epoxy value: 0.36mol / 100g.
Embodiment 2
[0029] step one
[0030] The side chain double bond of cardanol-thiol click chemical reaction: put 3.00g of cardanol and 1.72g of mercaptoethanol in a quartz flask, blow nitrogen, irradiate with ultraviolet light for 2 hours, and distill under reduced pressure at 90°C to get cashew nuts phenolic side chain double bond hydroxylation product II;
[0031] step two
[0032] Epoxidation reaction: 4.56g cardanol side chain double bond hydroxylation product II, 6.22g potassium carbonate and 52.94g dioxane were added together into a 100mL three-necked flask, the temperature was raised to 90°C by nitrogen gas, and the temperature was increased at 1000 rpm Stir at a speed of 1 / min, add 3.33g epichlorohydrin dropwise in 20 minutes, keep the temperature for 4 hours, filter, and the filtrate is distilled under reduced pressure to obtain the cardanol-based epoxy resin, epoxy value: 0.42mol / 100g.
Embodiment 3
[0034] step one
[0035] The side chain double bond of cardanol-thiol click chemical reaction: put 3.00g of cardanol and 2.34g of mercaptoethanol in a quartz flask, blow nitrogen, irradiate with ultraviolet light for 1 hour, and distill under reduced pressure at 80°C to get cashew nuts phenolic side chain double bond hydroxylation product III;
[0036] step two
[0037] Epoxidation reaction: Add 5.32g cardanol side chain double bond hydroxylation product III together with 8.29g potassium carbonate and 52.94g dioxane into a 100mL three-necked flask, heat up to 100°C with nitrogen gas, and Stir at a speed of 1 / min, add dropwise 4.44g epichlorohydrin in 30 minutes, keep warm for 6 hours, filter, and the filtrate is distilled under reduced pressure to obtain cardanol-based epoxy resin, epoxy value: 0.49mol / 100g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Epoxy value | aaaaa | aaaaa |
| Epoxy value | aaaaa | aaaaa |
| Epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com