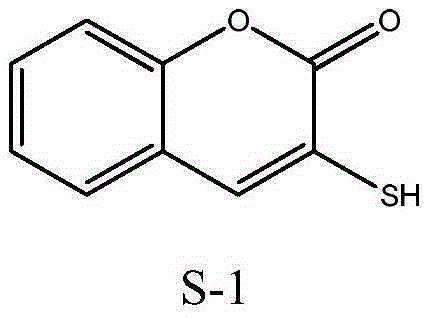
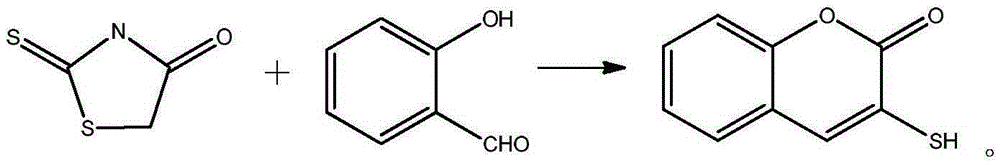
Method for preparing 3-sulfydryl coumarin
A technology of mercaptocoumarin and thioglycolic acid, applied in the direction of organic chemistry, can solve the problems of low yield, complicated post-processing, long reaction time, etc., and achieve the effects of low reaction temperature, low production cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation method of embodiment 1,3-mercaptocoumarin:
[0029] Under ice-bath condition, in the there-necked flask of 100ml, add 40.0g mass concentration and be the sodium hydroxide aqueous solution (0.30mol) of 30%, again in N 2 Add 11.0 g (0.12 mol) of thioglycolic acid under the protection of . After uniformly stirring for a period of time (about 20 to 30 minutes), add 14.0 g (0.10 mol) of o-chlorobenzaldehyde to the kettle liquid, raise the temperature to reflux temperature (T=108° C.), and reflux at this temperature for 4 hours. The reaction pressure is normal pressure. After the reaction, add concentrated hydrochloric acid (36.5wt%) to the still liquid until the still liquid is weakly acidic (i.e., adjust pH = 3 to 4), filter and dry (dry at 80°C for 10 h) to obtain 16.3 g of a yellow solid, which is a crude product 3-Mercaptocoumarin. The crude product was recrystallized with absolute ethanol (20ml), and after vacuum filtration, washing (washing with 30ml...
Embodiment 2
[0030] The preparation method of embodiment 2,3-mercaptocoumarin:
[0031] Under ice-bath condition, in the there-necked flask of 250ml, add 140.0g mass concentration and be 25% sodium hydroxide aqueous solution (0.875mol), again in N 2 26.7 g (0.29 mol) of thioglycolic acid were added under the protection of . After uniform stirring for a period of time, 35.1g (0.25mol) of o-chlorobenzaldehyde was added to the kettle liquid, and the temperature was raised to reflux temperature (T=106°C), and reflux reaction was carried out at this temperature for 4.5h, and the reaction pressure was normal pressure. After the reaction was completed, concentrated hydrochloric acid (36.5 wt %) was added to the still liquid until the still liquid was weakly acidic, and 41.2 g of a yellow solid was obtained by filtration and drying, which was the crude product 3-mercaptocoumarin. The crude product was recrystallized with absolute ethanol, and after vacuum filtration, washing and drying, 39.3 g of...
Embodiment 3
[0032] The preparation method of embodiment 3,3-mercaptocoumarin:
[0033] Under ice-bath condition, in the there-necked flask of 500ml, add 195.0g mass concentration and be 40% sodium hydroxide aqueous solution (1.95mol), again in N 2 71.9 g (0.78 mol) of thioglycolic acid were added under the protection of . After uniform stirring for a period of time, 84.3g (0.60mol) of o-chlorobenzaldehyde was added to the kettle liquid, and the temperature was raised to reflux temperature (T=112°C), and reflux reaction was carried out at this temperature for 3.5h, and the reaction pressure was normal pressure. After the reaction was finished, concentrated hydrochloric acid (36.5 wt %) was added to the still liquid until the still liquid was weakly acidic, and 98.4 g of a yellow solid was obtained by filtration and drying, which was the crude product 3-mercaptocoumarin. The crude product was recrystallized with absolute ethanol, and after vacuum filtration, washing and drying, 92.1 g of w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com