Protein D and HER2 fusion protein and preparing method and application thereof
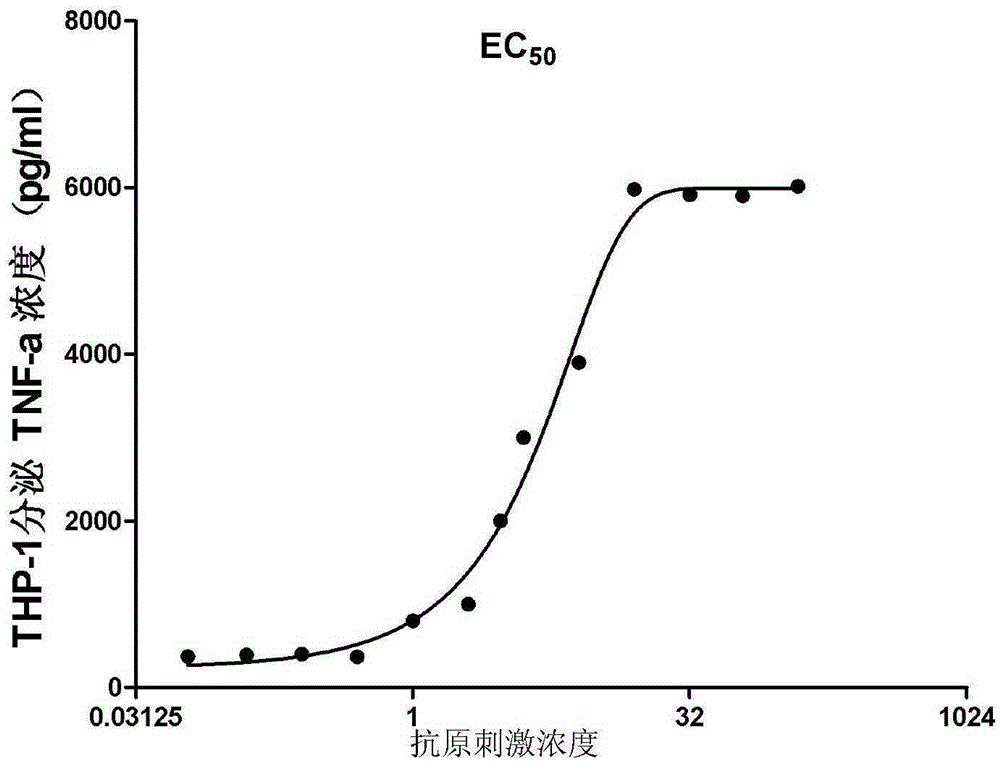
A technology of fusion protein and protein, which is applied in the fields of biology and medicine, can solve problems such as the impact of vaccine immunity, and achieve the effect of stimulating the body's immune response and delaying the growth rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
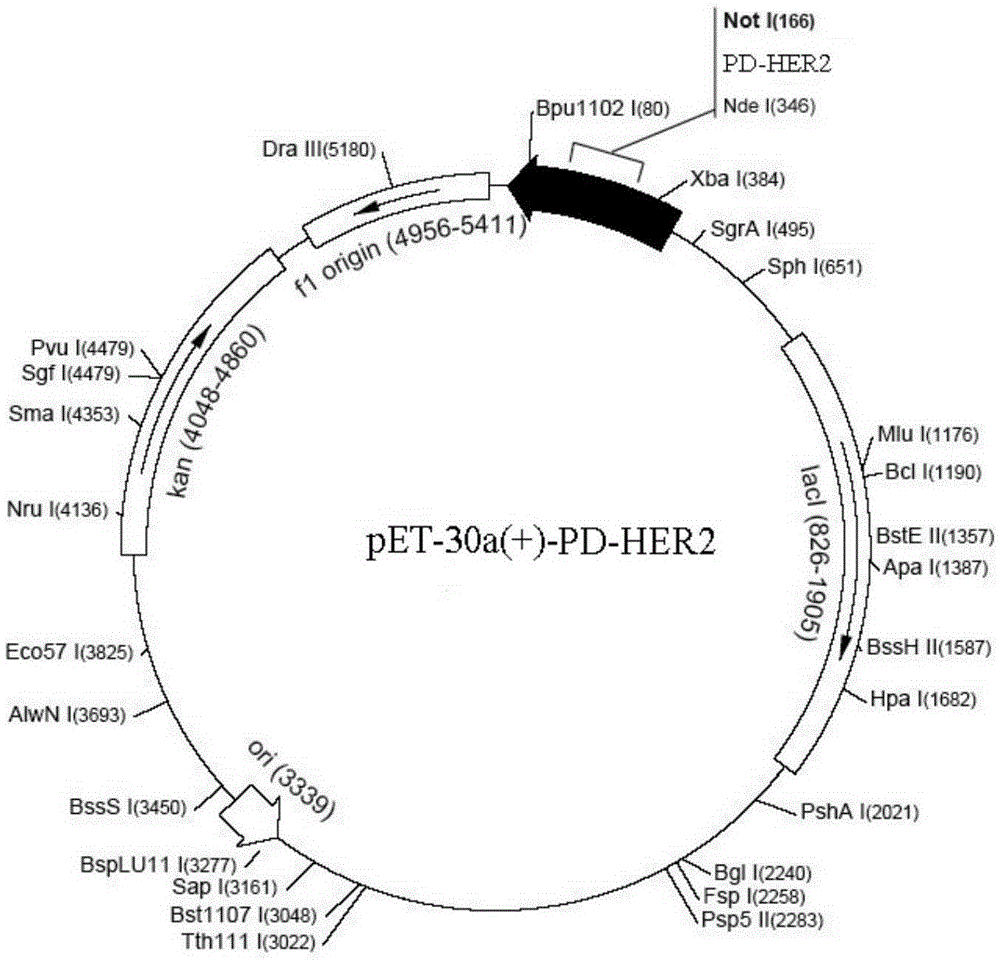
[0048] Example 1 Construction of recombinant PD-HER2 fusion protein therapeutic vaccine engineering bacteria
[0049] 1. Construction of pET30-a(+)-PD-HER2 / BL21(DE3) engineering bacteria
[0050] The PD protein derived from Hi (GenBank accession number: CAA84716) was selected, with a full length of 364 amino acids, as shown in SEQ ID NO:1. The 19-127 amino acids of PD protein are selected as the adjuvant protein, as shown in SEQ ID NO:2. Tumor-associated antigen human HER2 (GenBank accession number: AAA75493.1) has a full length of 1255 amino acids, as shown in SEQ ID NO:3. The HER2 antigen extracellular region fragment (41-560aa) and the HER2 epitope peptide fragment (774-788aa) are selected as vaccine molecular antigens. The amino acid sequence of the HER2 antigen extracellular region fragment (41-560aa) is shown in SEQ ID NO:4, and the HER2 epitope peptide fragment (774-788aa) is shown in SEQ ID NO:5.
[0051] The therapeutic vaccine molecule of the recombinant PD-HER2...
Embodiment 2
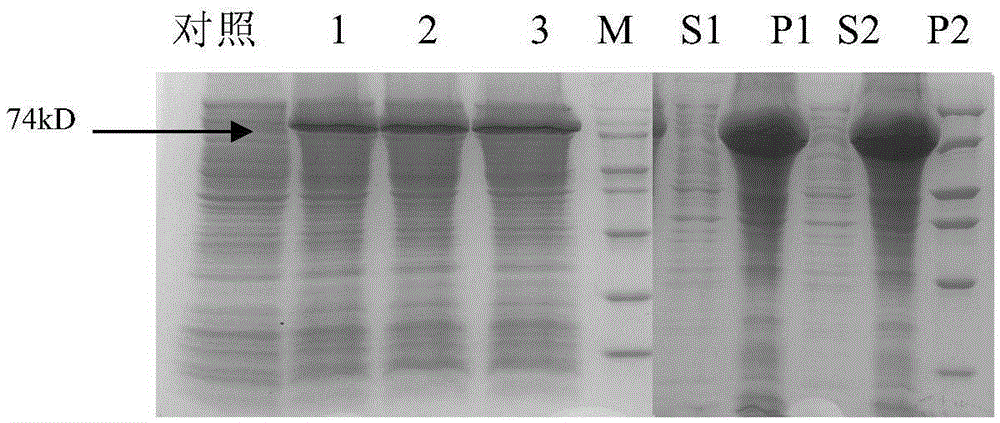
[0057] Example 2 Fermentation of recombinant PD-HER2 fusion protein engineering bacteria
[0058]The pET30-a(+)-PD-HER2 / BL21(DE3) engineering bacteria constructed in the above Example 1 were streaked LB plates (kan100mg / L) and cultured in a 37°C constant temperature incubator (Bo Xun, Shanghai) for about 16- 18h, until a single colony grows. A single colony of engineered bacteria was picked and inoculated into 20ml of LB medium (kan100mg / L), and cultured at 37°C and 230rpm for 8h. 0.1% was transferred to 250ml LB (kan100mg / L), 1L Erlenmeyer flask, cultured at 37°C, 230rpm for 13h. Four bottles were cultured in parallel, 1000ml of bacterial solution was prepared, and 5% was inoculated into the medium of the upper tank of the fermenter NLF-2220L. Before inoculation, the pH was adjusted to 7.0 with ammonia water, and the temperature during the fermentation process was controlled at 36°C. The pH value and dissolved oxygen of the culture medium were controlled by adding ammonia...
Embodiment 3
[0060] Example 3 Separation and purification of recombinant PD-HER2 fusion protein
[0061] 1. Inclusion body washing
[0062] (1) First wash
[0063] Take the precipitate collected in Example 2, the main component is inclusion body (IB). Wash with washing buffer (20mM Tris-HCl, 2M urea (Sinopharm, Shanghai), 1% TritonX-100 (sigma, USA), 5mM EDTA (Sinopharm, Shanghai), 0.2M NaCl (Sinopharm, Shanghai), pH8.0). IB: washing buffer = 1g: 20ml, at room temperature, magnetically stirred for 30min, centrifuged at 8000g for 10min to remove the supernatant.
[0064] (2) Second wash
[0065] Inclusion bodies collected after the first wash were precipitated and washed with wash buffer (20 mM Tris-HCl, 2M urea, 5 mM EDTA, 0.2M NaCl, pH 8.0). IB: washing buffer = 1g: 20ml, at room temperature, magnetically stirred for 30min, centrifuged at 8000g for 10min to remove the supernatant.
[0066] (3) The third wash
[0067] Inclusion bodies collected after the third wash were precipitated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com