Method for in-situ preparation of iron carbide filled doped carbon nanotube
A technology for in-situ preparation of carbon nanotubes, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the cumbersome preparation process, low precursor utilization rate, low iron utilization rate, etc. problem, to achieve the effect of high utilization rate, low precursor cost, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of iron carbide filled nitrogen-doped carbon nanotubes:
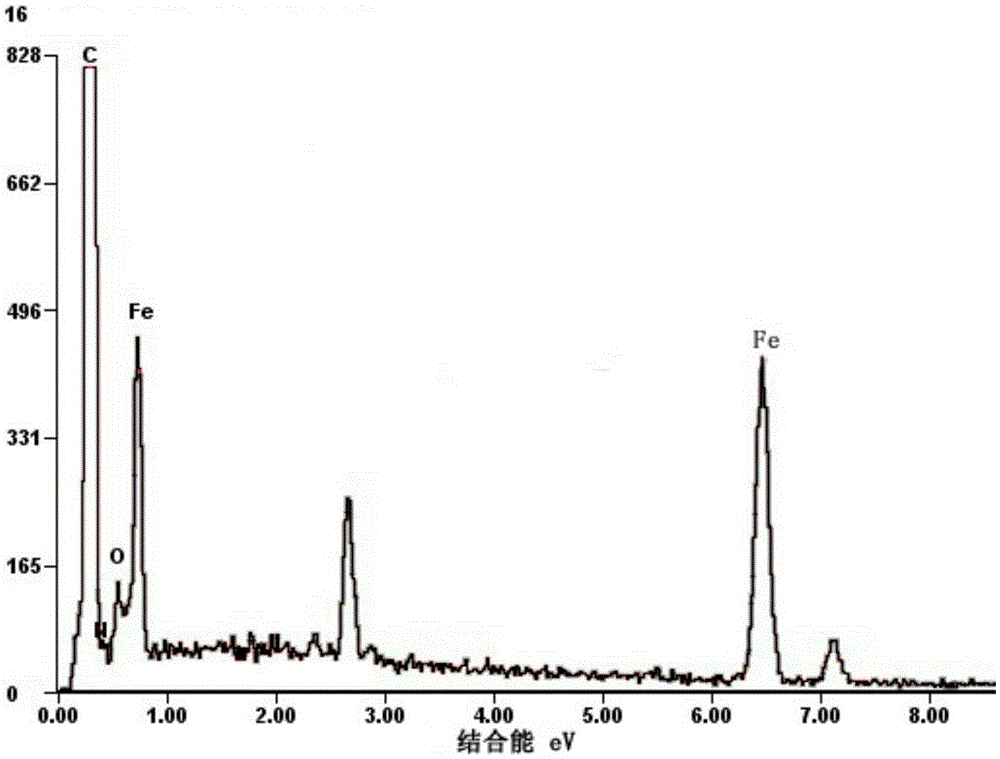
[0037] Dissolve and disperse 1.5g of ferric chloride hexahydrate and 1.5g of melamine in 100mL of ethanol solution. After ultrasonication for half an hour, put them in an oven at 110°C for 12h. Grind the dried mixed powder evenly, put it into a porcelain boat, and put it in the high temperature zone of the tube furnace. Introduce Ar into the tube furnace at a rate of 160 mL / min, raise the temperature to 800 °C at a rate of 10 °C / min, keep it for 2 h, and naturally cool to room temperature to obtain a black powder. The black powder was pickled in 200mL, 2mol / L hydrochloric acid solution for 6h, filtered and dried to obtain nitrogen-doped carbon nanotubes filled with iron carbide. The weight of the sample was 0.4g, and the filling amount of iron was about 27%.
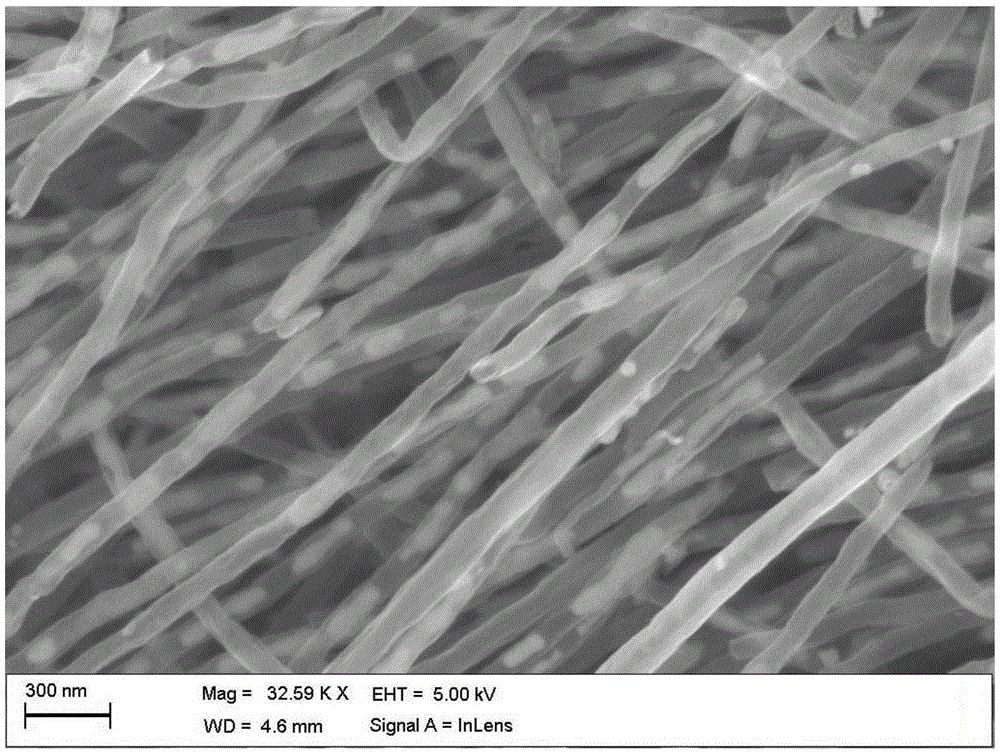
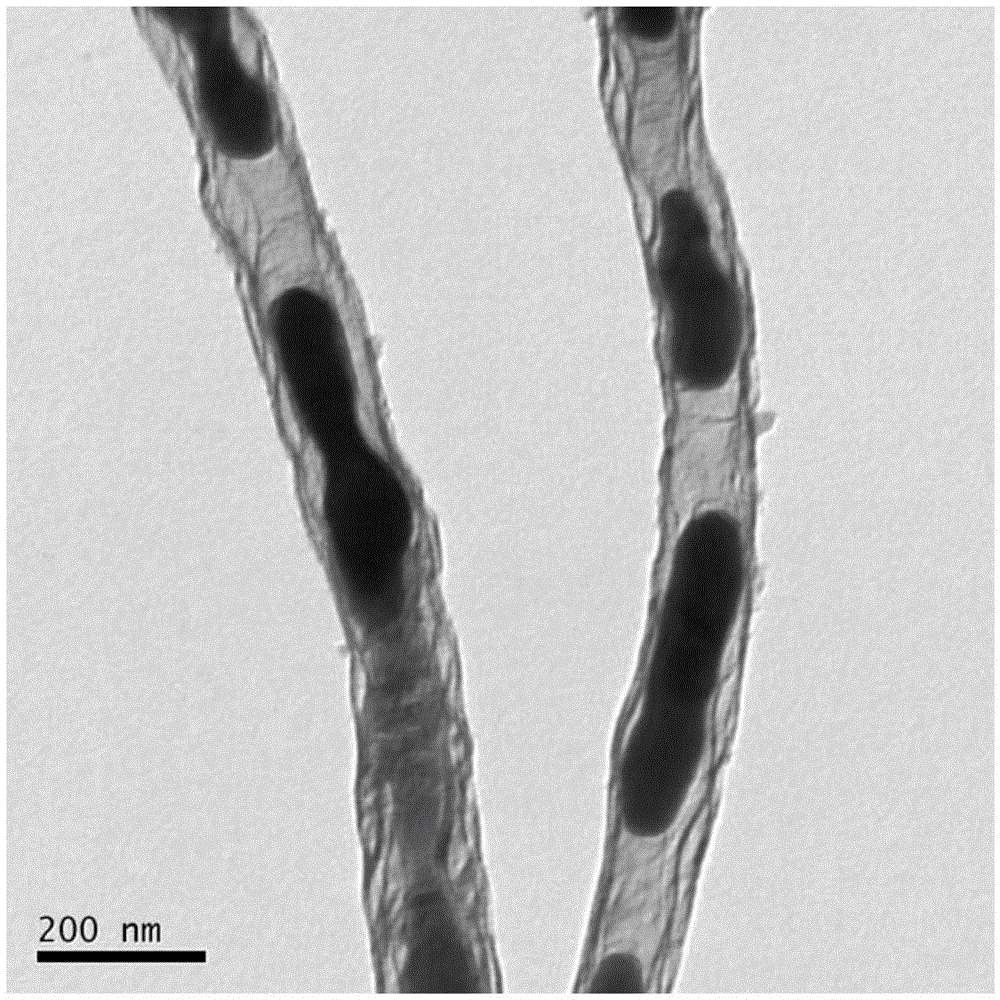
[0038]The SEM image of the obtained iron carbide filled nitrogen-doped carbon nanotube sample is shown in figure 1 shown, from figure 1 It c...
Embodiment 2
[0042] Preparation of iron carbide-filled nitrogen-boron co-doped carbon nanotubes:
[0043] Dissolve 1.5g of ferric chloride hexahydrate, 1.5g of melamine, and 0.2g of boric acid in 100ml of ethanol solution, ultrasonicate for half an hour, and dry in an oven at 110°C for 12h. Grind the dried mixed powder evenly, put it into a porcelain boat, and put it in the high temperature zone of the tube furnace. Introduce Ar into the tube furnace at a rate of 160 mL / min, raise the temperature to 800 °C at a rate of 10 °C / min, keep it for 2 h, and naturally cool to room temperature to obtain a black powder. The black powder was pickled in 200mL, 2mol / L hydrochloric acid solution for 6 hours, filtered and dried to obtain iron carbide-filled nitrogen-boron-doped carbon nanotubes. The weight of the sample was 0.4g, and the iron filling amount was about 20%.
[0044] The SEM image of the obtained iron carbide filled nitrogen-boron co-doped carbon nanotube sample is as follows Figure 6 As...
Embodiment 3
[0046] Preparation of iron carbide filled nitrogen and phosphorus co-doped carbon nanotubes:
[0047] Dissolve and disperse 0.5g of ferric chloride hexahydrate, 1.5g of melamine, and 0.2mL of phosphoric acid in 100ml of ethanol solution. After ultrasonication for half an hour, dry in an oven at 110°C for 12h. Grind the dried mixed powder evenly, put it into a porcelain boat, and put it in the high temperature zone of the tube furnace. Introduce Ar into the tube furnace at a rate of 160 mL / min, raise the temperature to 900 °C at a rate of 10 °C / min, keep it for 2 h, and naturally cool to room temperature to obtain a black powder. The black powder was pickled in 200mL, 2mol / L hydrochloric acid solution for 6h, filtered and dried to obtain iron carbide-filled nitrogen-phosphorus-doped carbon nanotubes. The weight of the sample was 0.4g, and the iron filling amount was about 20%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com