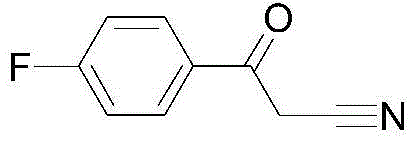
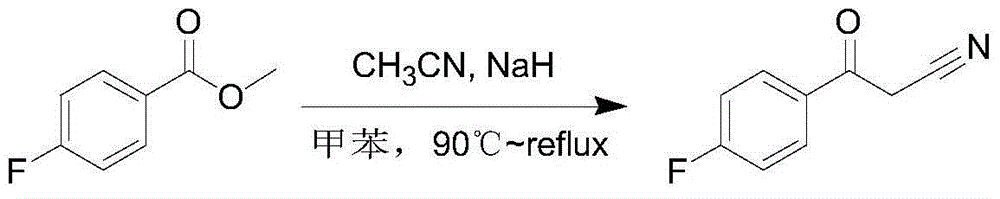Preparation method for high-purity 4-fluorobenzoylacetonitrile
A -fluorobenzoylacetonitrile, high-purity technology, applied in the field of preparation of high-purity 4-fluorobenzoylacetonitrile, can solve the problems of low yield, difficult purification, large impurities, etc., and achieves a simple purification method and easy industrialization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add anhydrous tetrahydrofuran (100mL) to a 500mL three-necked flask, add sodium (3.14g), under nitrogen protection, cool to -30°C, add acetonitrile (25mL) dropwise, the temperature does not exceed 0°C, the dropwise addition is completed, At -30-0°C, the sodium reaction is complete, add dropwise a solution of 4-fluorobenzoic acid methyl ester (20g) in tetrahydrofuran (50mL), the temperature is between -30-0°C, the dropwise addition is complete, and the reaction is in this temperature range Continue the reaction in middle temperature, TLC plate monitoring, the raw material reaction is complete, dropwise add methanol (20mL) to quench the reaction, the temperature does not exceed 0 °C, the quenching is complete, adjust the pH value to 7 with 1N hydrochloric acid, separate the liquid, and use ethyl acetate for the aqueous phase Extraction, the organic phase was washed with saturated salt, dried with anhydrous sodium sulfate, concentrated to dryness under reduced pressure, and...
Embodiment 2
[0025] Add acetonitrile (100mL) into a 250mL three-neck flask, lower the temperature to -30°C, and add sodium (3.28g) in batches under nitrogen protection, the temperature does not exceed 0°C, the sodium reaction is complete, drop 4-fluorine Methyl benzoate (20g) in acetonitrile (50mL) solution, the temperature is between -30 ~ 0 ℃, the dropwise addition is completed, the reaction continues to react in this temperature range, TLC board monitoring, the raw material reaction is complete, dropwise add methanol (20mL ) to quench the reaction, the temperature does not exceed 0°C, the quenching is complete, the pH value is adjusted to 7 with 1N hydrochloric acid, the liquid is separated, the aqueous phase is extracted with ethyl acetate, the organic phase is washed with saturated salt, and dried with anhydrous sodium sulfate. Concentrate to dryness under reduced pressure, and beat with petroleum ether to obtain 20.1 g of white solid, HPLC: 99.3%, excluding 4‐methoxybenzoyl acetonitri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com