Method for extracting and separating valuable metal from secondary lead smelting slag
A technology for secondary lead smelting and valuable metals, applied in the direction of improving process efficiency, etc., can solve the problems of acid mist, loss of alkali solution, poor operating environment, etc., and achieve the effect of easy operation, simple process, and improved recycling rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
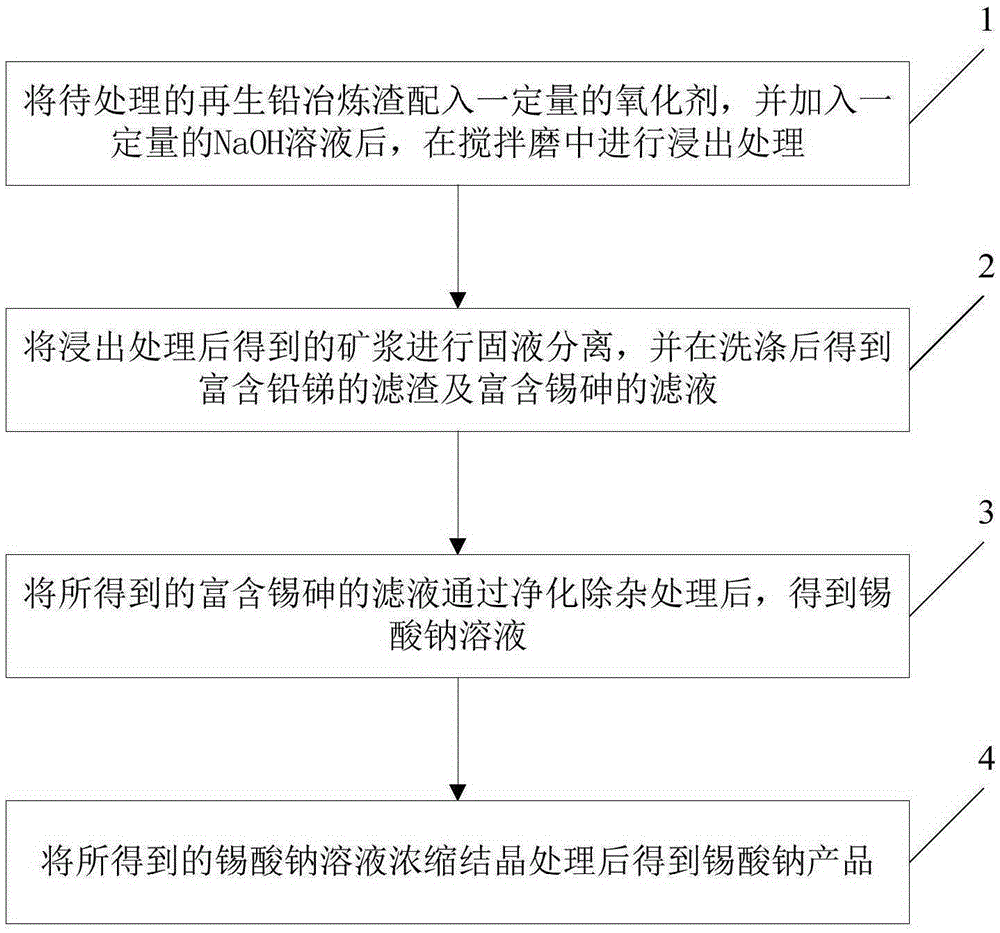
[0034] Embodiment 1, mix the regenerated lead smelting slag containing 33% lead, 9.81% tin, 6.92% antimony, and 4.28% arsenic into 5% lead paste, add a certain amount of NaOH, and directly carry out stirring mill leaching, and the leaching temperature is 60 ℃, the time is 2h, the liquid-solid ratio is 2.5:1mL / g, the stirring speed is 200rpm, NaOH150g / L, the leaching rate of tin is 85%, the leaching rate of arsenic is 90%, the leaching rate of lead is as low as 7%, and the leaching rate of antimony As low as 0.1%, leach residue and leachate can be obtained after filtering and washing;
[0035] The leaching residue is lead-antimony leaching residue, which contains more than 76% lead, more than 12% antimony, less than 2% tin, and less than 1% arsenic; L;
[0036] The tin-rich leaching solution is purified to remove impurities to obtain a sodium stannate solution with lead and arsenic less than 0.0005 g / L, and after concentration and crystallization, a sodium stannate product wit...
Embodiment 2
[0037] Example 2, mix the regenerated lead smelting slag containing 33% lead, 9.81% tin, 6.92% antimony, and 4.28% arsenic into 3% hydrogen peroxide, add a certain amount of NaOH, and directly carry out stirring mill leaching, and the leaching temperature is 80°C , the time is 2 hours, the liquid-solid ratio is 3:1mL / g, the stirring speed is 400rpm, NaOH120g / L, the leaching rate of tin is 88%, the leaching rate of arsenic is 89%, the leaching rate of lead is 10%, and the leaching rate of antimony is as low as 0.1%. , after filtering and washing, leach residue and leachate are obtained;
[0038] The leaching slag is lead-antimony leaching slag, containing more than 74% lead, more than 12% antimony, less than 2% tin, and less than 1% arsenic; the leaching solution is tin-rich arsenic leaching solution, containing 22g / L tin, 9g / L lead, and 9g / L arsenic L;
[0039] The tin-rich leaching solution is purified to remove impurities to obtain a sodium stannate solution with less than ...
Embodiment 3
[0040] Example 3, mix the regenerated lead smelting slag containing 33% lead, 9.81% tin, 6.92% antimony, and 4.28% arsenic into 10% lead paste, hydrogen peroxide and sodium peroxide mixture, add a certain amount of NaOH, and directly stir Mill leaching, the leaching temperature is 90°C, the time is 3h, the liquid-solid ratio is 5:1mL / g, the stirring speed is 300rpm, NaOH120g / L, among which the tin leaching rate is 90%, the arsenic leaching rate is 94%, and the lead leaching rate is 10%. , the leaching rate of antimony is as low as 0.1%, and the leaching residue and leachate are obtained after filtering and washing;
[0041] The leaching slag is lead-antimony leaching slag, containing more than 74% lead, more than 12% antimony, less than 2% tin, and less than 1% arsenic; the leaching solution is tin-rich arsenic leaching solution, containing 23g / L tin, 9g / L lead, and 11g / L arsenic. L;
[0042] The tin-rich leaching solution is purified to remove impurities to obtain a sodium s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com