Method for producing sodium fluoride from lithium source lithium extraction waste liquid and method for co-producing sodium fluoride and potassium fluosilicate
A technology of potassium fluorosilicate and sodium fluoride, which is applied in the field of waste liquid treatment of lithium extraction from lithium sources, can solve the problems of low development and utilization value of associated resources and difficult disposal of waste liquid, and achieve good economic and environmental benefits, reducing Emissions, the effect of saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
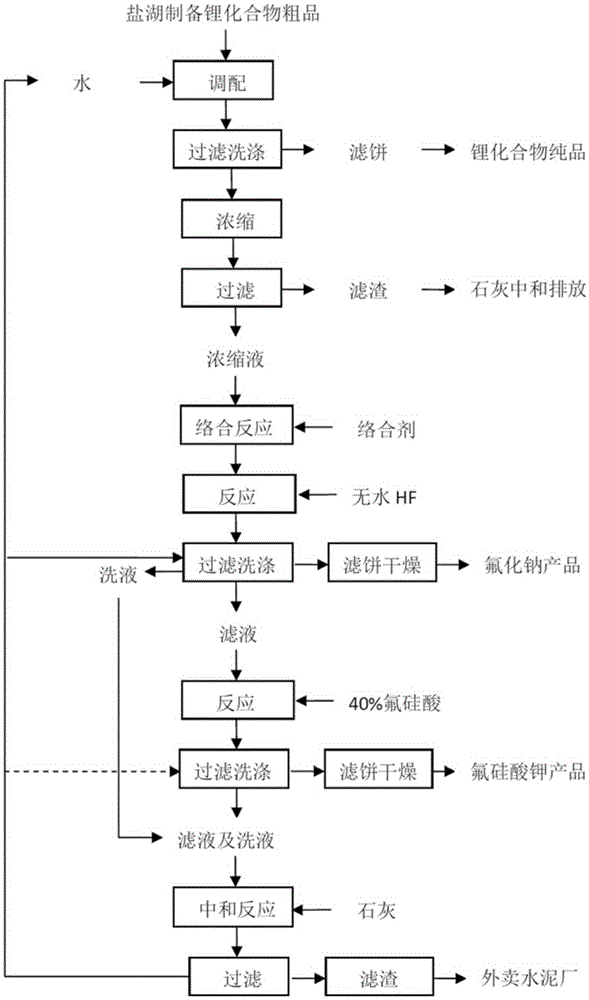
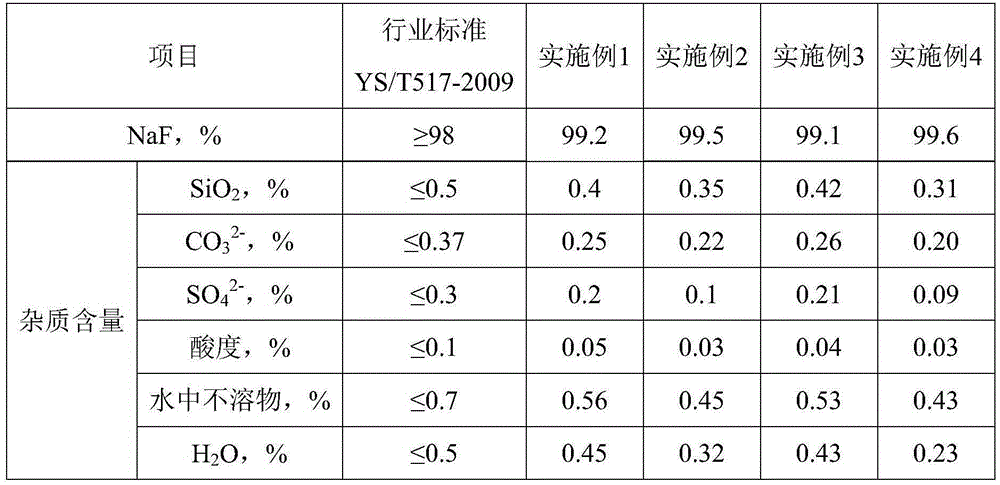
[0051] The method for producing sodium fluoride and co-producing potassium fluorosilicate by utilizing lithium source to extract lithium waste liquid of the present embodiment comprises the following steps:
[0052] 1) Add 1000g of crude lithium extracted from salt lake (crude lithium compound prepared by salt lake) to 5000g of water (the mass ratio of liquid to solid is 5:1), stir at room temperature for 5h to make a slurry, filter, wash to obtain 585g of lithium concentrate, and combine The filtrate and washing liquid are used to extract lithium waste liquid; the obtained lithium concentrate is used to prepare high-purity lithium compounds; the Na+ mass concentration in the obtained lithium extraction waste liquid is 14.5%;
[0053] 2) 5415g, containing Na + Add 1g of sodium ethylenediaminetetraacetate as a complexing agent to the lithium extraction waste liquid with a mass concentration of 14.5% (the mass ratio of the complexing agent to the impurity metal ions in the lithi...
Embodiment 2
[0059] The method of using the lithium source to extract lithium waste liquid of the present embodiment to produce sodium fluoride and co-produce potassium fluorosilicate, such as figure 1 shown, including the following steps:
[0060] 1) Add 1000g of crude lithium extracted from salt lake (crude lithium compound prepared by salt lake) to 5000g of water (the mass ratio of liquid to solid is 5:1), stir at room temperature for 10h to make a slurry, filter, wash to obtain 940g of lithium concentrate, and combine The filtrate and washing liquid are used to extract lithium waste liquid; the obtained lithium concentrate is used to prepare high-purity lithium compounds; the Na+ mass concentration in the obtained lithium extraction waste liquid is 7%;
[0061] 2) Concentrate 5060g of the lithium extraction waste liquid containing Na+mass concentration of 7% obtained in step 1) under a negative pressure of less than 0.1MPa and 40°C, concentrate 2615g of evaporated water until the Na+ma...
Embodiment 3
[0068] The method for producing sodium fluoride and co-producing potassium fluorosilicate by utilizing lithium source to extract lithium waste liquid of the present embodiment comprises the following steps:
[0069] 1) add water (the mass ratio of liquid to solid is 1:1) to the crude lithium extracted from salt lake (the crude lithium compound prepared from salt lake), stir for 15 hours to make a slurry, filter, wash to obtain lithium concentrate, and combine the filtrate and washing liquid to obtain Lithium extraction waste liquid; the obtained lithium concentrate is used to prepare high-purity lithium compounds; the Na+ mass concentration in the obtained lithium extraction waste liquid is 10%;
[0070] 2) Concentrate the lithium extraction waste liquid containing 10% Na+ mass concentration obtained in step 1) under a negative pressure of 0.06MPa and 60°C until the Na+ mass concentration is 15%, and then filter at 60°C to obtain Lithium extraction waste liquid concentrate and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com