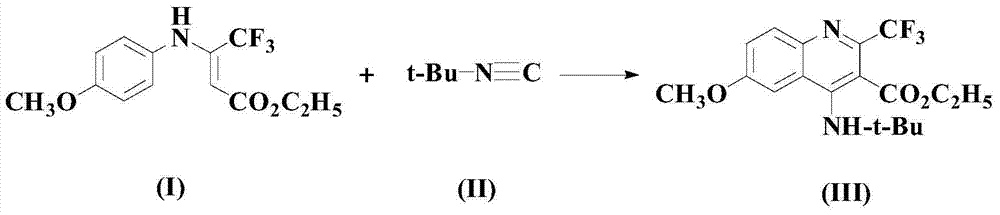
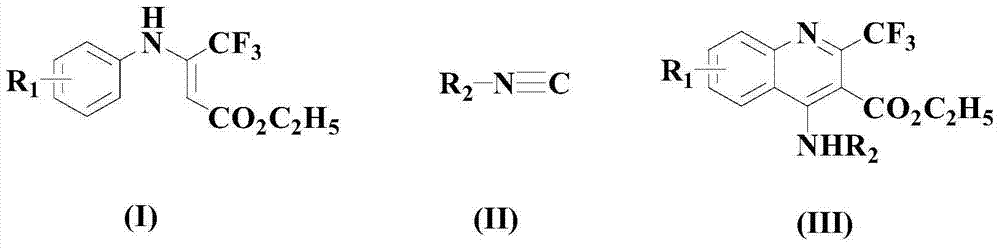A kind of synthetic method of pharmaceutical intermediate 4-aminoquinoline compounds
A synthesis method and aminoquinoline technology, applied in the direction of organic chemistry and the like, can solve the problems such as unreported cyclization synthesis of quinoline compounds, and achieve the effect of good application prospect, industrialization potential and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
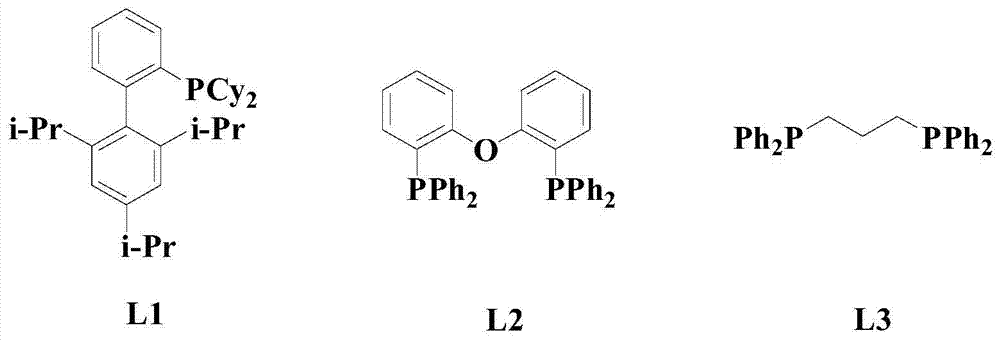
[0042] Under nitrogen atmosphere, add 100mmol formula (I) compound and 150mmol formula (II) compound to solvent dichloromethane, stir and mix for 10 minutes, then add 6mmol catalyst PdCl successively 2 (MeCN) 2 , 100mmol additive CuBr 2 , 220mmol base NaHCO 3 , 4mmol of ligand L1 and 10mmol of additives, heated up to 50°C and stirred for 5 hours, cooled to room temperature after the reaction, concentrated in vacuo, and passed through 300-400 mesh silica gel column chromatography (with ethyl acetate in a volume ratio of 1:2 The mixed solution with acetone was used as the eluent) to obtain the compound of formula (III) (wherein t-Bu is tert-butyl) with a yield of 97.3%.
[0043] Wherein, the auxiliary agent is a mixture of polyethylene glycol 400 and butyltriphenylphosphine bromide, wherein the molar ratio of polyethylene glycol 400 to butyltriphenylphosphine bromide is 1:2.
[0044] 1 H NMR (CDCl 3 ,400MHz):δ8.03(d,J=9.2Hz,1H),7.66(s,1H),7.43(d,J=9.1Hz,1H),4.87...
Embodiment 2
[0047]
[0048] Under nitrogen atmosphere, add 100mmol formula (I) compound and 200mmol formula (II) compound to solvent m-dichlorobenzene, stir and mix for 12 minutes, then add 7mmol catalyst PdCl successively 2 (MeCN) 2 , 150mmol additive CuBr 2 , 240mmol base NaHCO 3 , 7mmol of ligand L1 and 12mmol of additives, heated up to 55°C and stirred for 4.5 hours, cooled to room temperature after completion of the reaction, concentrated in vacuo, and passed through 300-400 mesh silica gel column chromatography (with ethyl acetate in a volume ratio of 1:2 (mixture with acetone as eluent) was purified to obtain the compound of formula (III) (wherein t-Bu is tert-butyl) with a yield of 97.7%.
[0049] Wherein, the auxiliary agent is a mixture of polyethylene glycol 400 and butyltriphenylphosphine bromide, wherein the molar ratio of polyethylene glycol 400 to butyltriphenylphosphine bromide is 1:2.5.
[0050] 1 H NMR (CDCl 3 ,400MHz):δ8.28(d,J=12.0Hz,1H),8.14(s,1H),7.53(d,J=12....
Embodiment 3
[0053]
[0054] Under nitrogen atmosphere, add 100mmol formula (I) compound and 250mmol formula (II) compound to solvent chloroform, stir and mix for 15 minutes, then add 8mmol catalyst PdCl successively 2 (MeCN) 2 , 200mmol additive CuBr 2 , 260mmol base NaHCO 3 , 10mmol of ligand L1 and 15mmol of additives, heated up to 60°C and stirred for 5 hours, cooled to room temperature after the reaction, concentrated in vacuo, and passed through 300-400 mesh silica gel column chromatography (with ethyl acetate in a volume ratio of 1:2 (mixture with acetone as eluent) was purified to obtain the compound of formula (III) (wherein t-Bu is tert-butyl) with a yield of 98.1%.
[0055] Wherein, the auxiliary agent is a mixture of polyethylene glycol 400 and butyltriphenylphosphine bromide, wherein the molar ratio of polyethylene glycol 400 to butyltriphenylphosphine bromide is 1:3.
[0056] 1 H NMR (CDCl 3 ,400MHz):δ8.23(d,J=8.0Hz,1H),7.62(d,J=8.0Hz,1H),7.46(t,J=8.0Hz,1H),5.04(s,1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com