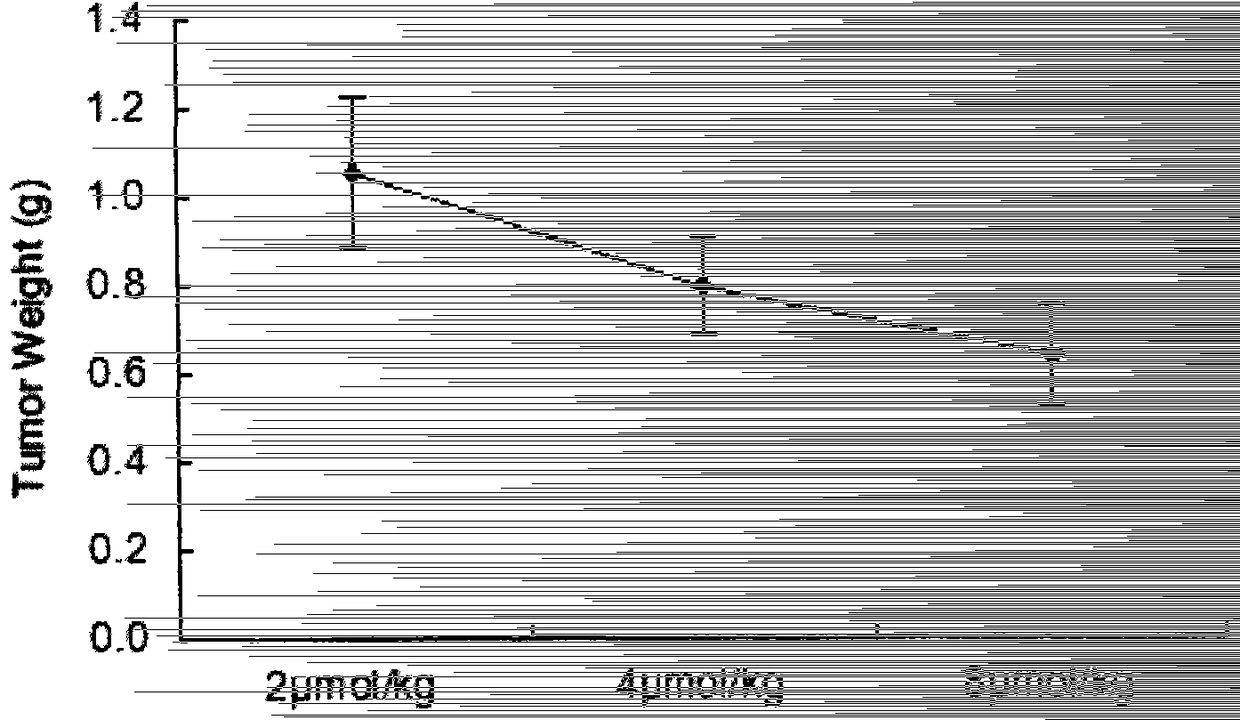
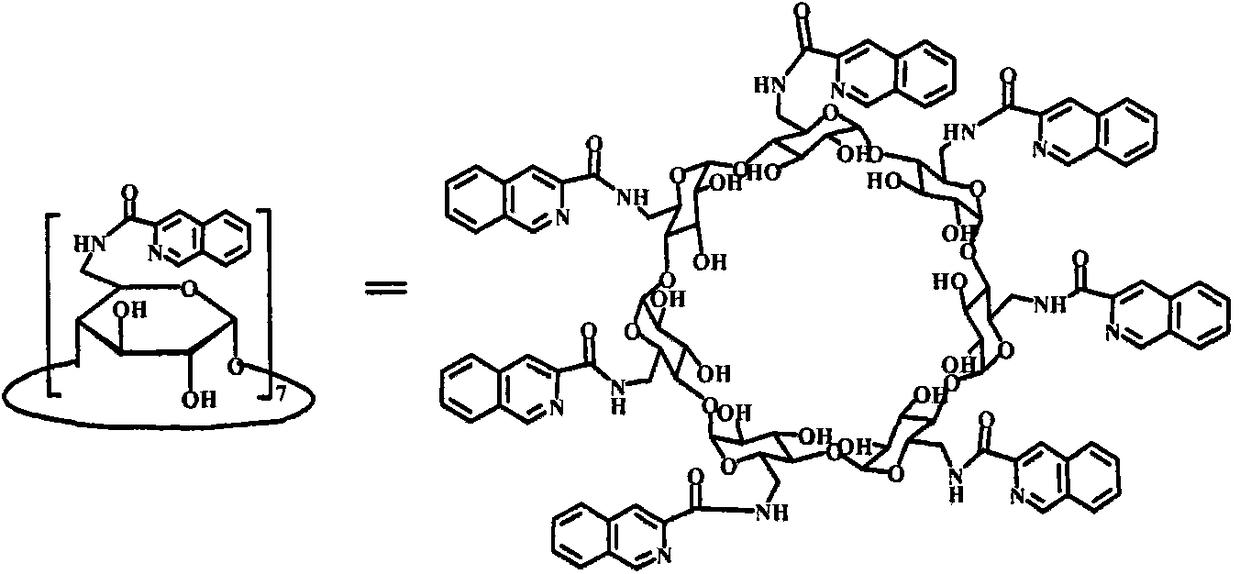
Hepta(6-(isoquinoline-3-amido)-6-deoxy)-β-cyclodextrin and its preparation method and application
A kind of technology of cyclodextrin and isoquinoline, which is applied in the field of biomedicine and can solve the problems such as antitumor activity of uninstructed compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
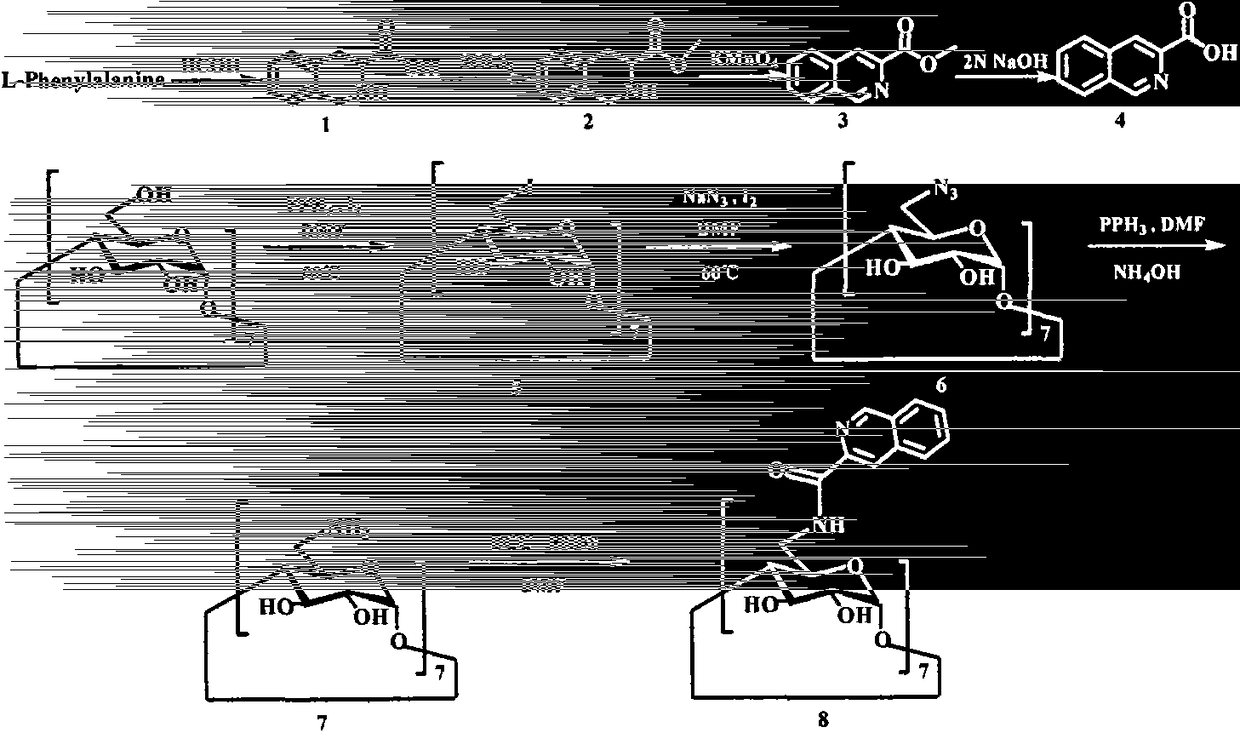
[0017] Example 1 Preparation of (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (1)
[0018] Put HCl (320ml) and HCHO (160ml) in a 500ml eggplant bottle, add L-Phe (30.10g, 182.45mmol) after mixing, react in an oil bath at 95°C for 24h, stop the reaction, and cool to room temperature. The pH was adjusted to 6-7 with saturated NaOH in an ice bath, and a large amount of white solids precipitated out. Filtration gave a white solid (21.80 g, 67.54%). ESI-MS(m / z): 178.1[M+H] + .
Embodiment 2
[0019] Example 2 Preparation of (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid methyl ester (2)
[0020] Add methanol (100ml) to a 250ml eggplant bottle, add thionyl chloride (15ml) drop by drop in an ice-salt bath, stir for 30min, then slowly add (3S)-1,2,3,4-tetrahydroisoquinoline -3-Carboxylic acid (1) (10.00g, 56.50mmol), reacted for 9-10h, and the reaction solution was drained to obtain a white solid (8.46g, 78.40%). ESI-MS(m / z): 192.0[M+H] + .
Embodiment 3
[0021] Example 3 Preparation of methyl isoquinoline-3-carboxylate (3)
[0022] Add (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid methyl ester (2) (4.23g, 22.15mmol) in a 250ml eggplant bottle, add N,N- Dissolve it in dimethylformamide (50ml), add 2.97g (18.79mmol) of potassium permanganate, react for 6h, add ethanol (50ml) and stir for 30min, remove the solvent, add methanol (30ml) to redissolve. Separation and purification by column chromatography gave a colorless solid (858mg, 24.56%). ESI-MS(m / z): 188.0[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com