Separation and detection method of glycyl-L-glutamine chiral isomer
A chiral isomer, glutamine technology, applied in the field of analytical chemistry, can solve the problems of difficult separation, high finished product, weak ultraviolet absorption, etc., and achieve the effects of high analytical sensitivity, stable product, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
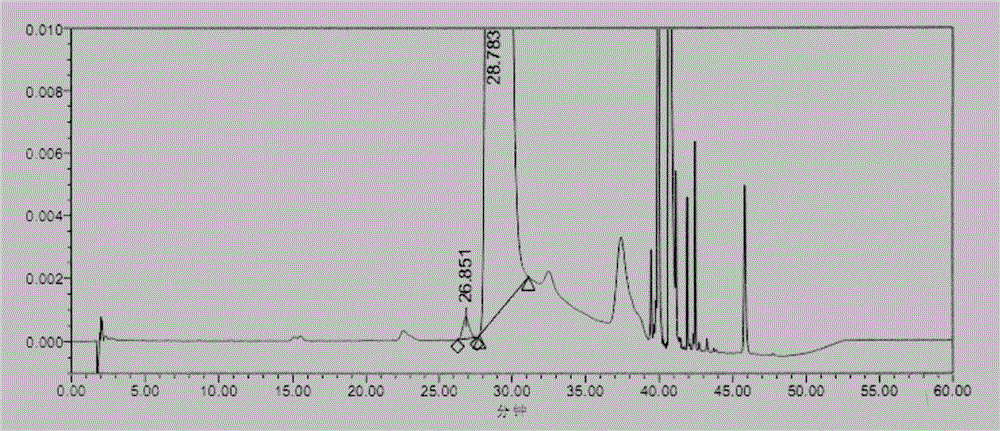
Embodiment 1
[0037] (1) Instruments and conditions
[0038] Chromatographic conditions:
[0039] Chromatographic column: base-passivated octadecyl-bonded silica gel column (4.6mm×250mm, 5μm)
[0040] Mobile phase: Phase A: 0.09% trifluoroacetic acid (adjust pH 2.3 with NaOH)-acetonitrile (94:6)
[0041] Phase B: Acetonitrile
[0042] Detection wavelength: 340nm; column temperature: 25°C; flow rate: 1.6ml / min; diluent: water
[0043] Gradient elution program:
[0044]
[0045] (2) Experimental steps
[0046] Derivatization test solution: take Merfey's [N α -(5-fluoro-2,4-dinitrophenyl)-L-alanamine amide] reagent amount, accurately weighed, add acetone to dissolve and dilute to make a 10mg / ml solution, shake well, and place in the refrigerator ( 2℃~8℃) for storage.
[0047] Sample solution: Take an appropriate amount of glycyl-L-glutamine and its isomers, dissolve and dilute with water to make a mixed solution containing about 30mg of glycyl-L-glutamine and 30μg of isomers per 1ml,...
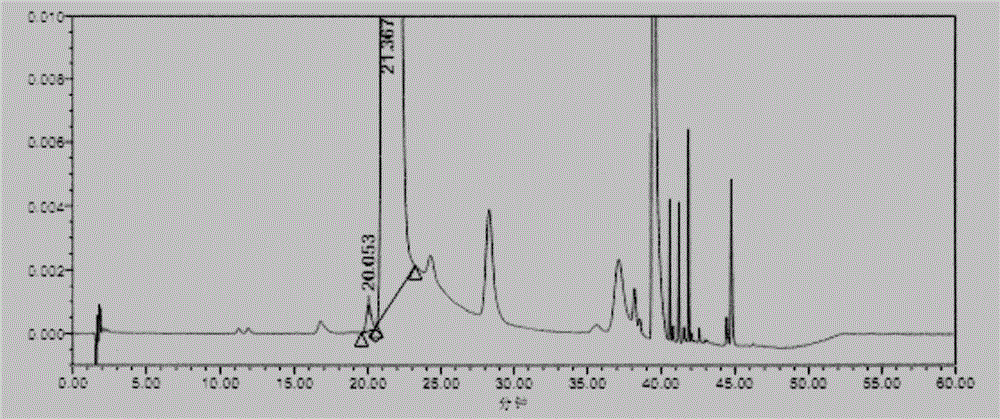
Embodiment 2
[0051] (1) Instruments and conditions
[0052] Chromatographic conditions:
[0053] Chromatographic column: base-passivated octadecyl-bonded silica gel column (4.6mm×250mm, 5μm)
[0054] Mobile phase: Phase A: 0.11% trifluoroacetic acid (adjust pH 2.7 with NaOH)-acetonitrile (86:14)
[0055] Phase B: Acetonitrile
[0056] Detection wavelength: 340nm; column temperature: 35°C; flow rate: 2.0ml / min; diluent: water
[0057] Gradient elution program:
[0058]
[0059] (2) Experimental steps
[0060] Derivatization test solution: take Merfey's [N α -(5-fluoro-2,4-dinitrophenyl)-L-alanamine amide] reagent amount, accurately weighed, add acetone to dissolve and dilute to make a 10mg / ml solution, shake well, and place in the refrigerator ( 2℃~8℃) for storage.
[0061] Sample solution: take appropriate amount of glycyl-L-glutamine and glycyl-D-glutamine, dissolve and dilute with water to make about 30mg of glycyl-L-glutamine, glycyl-D-glutamine per 1ml - A mixed solution of 30...
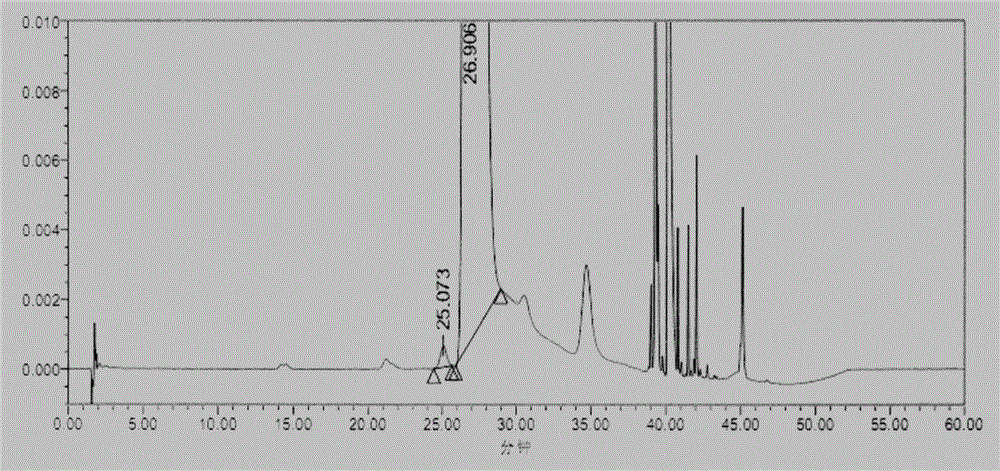
Embodiment 3
[0065] (1) Instruments and conditions
[0066] Chromatographic conditions:
[0067] Chromatographic column: base-passivated octadecyl-bonded silica gel column (4.6mm×250mm, 5μm)
[0068] Mobile phase: Phase A: 0.1% trifluoroacetic acid (adjust pH 2.5 with NaOH)-acetonitrile (88:12)
[0069] Phase B: Acetonitrile
[0070] Detection wavelength: 335nm; column temperature: 30°C; flow rate: 1.8ml / min; diluent: water
[0071] Gradient elution program:
[0072]
[0073] (2) Experimental steps
[0074] Derivatization test solution: take Merfey's [N α -(5-fluoro-2,4-dinitrophenyl)-L-alanamine amide] reagent amount, accurately weighed, add acetone to dissolve and dilute to make a 10mg / ml solution, shake well, and place in the refrigerator ( 2℃~8℃) for storage.
[0075] Sample solution: take appropriate amount of glycyl-L-glutamine and glycyl-D-glutamine, dissolve and dilute with water to make about 30mg of glycyl-L-glutamine, glycyl-D-glutamine per 1ml - A mixed solution of 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com