Mucus layer-adhesive poly gamma-glutamic acid nanomicelles and drug delivery system using same
A technology of nano micelles and glutamic acid, which can be applied in the fields of nano-drugs, nano-technology, nano-technology, etc., can solve the problems of difficult mucosal layer and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of poly-γ-glutamic acid / cholesterol nanomicelles
[0048] Synthesis of cholesteryl-amine
[0049] 250 mmol of ethylenediamine (Sigma-Aldrich, USA) was dissolved in 250 ml of toluene. At this point, the temperature was kept low with ice. 2.25g of cholesterol was dissolved in 50ml of toluene, and after standing for 10 minutes, it was added dropwise to the above-prepared ethylenediamine solution for mixing, and immediately stirred at room temperature for 16 hours to carry out the reaction. After the reaction, it was washed several times with deionized water. The organic layer which became clear through the above process was dried with magnesium sulfate, and the toluene was rotovapped from the dried solution. The evaporated sample was rinsed several times with a mixed solution of 20 ml of dichloromethane and 20 ml of methanol, and filtered with a 1 micron PTFE filter. The filtered and clear solution was re-rotoevaporated to yield a sample of cholesteryl-am...
Embodiment 2
[0055] Characterization of poly-γ-glutamic acid-cholesterol nanomicelles
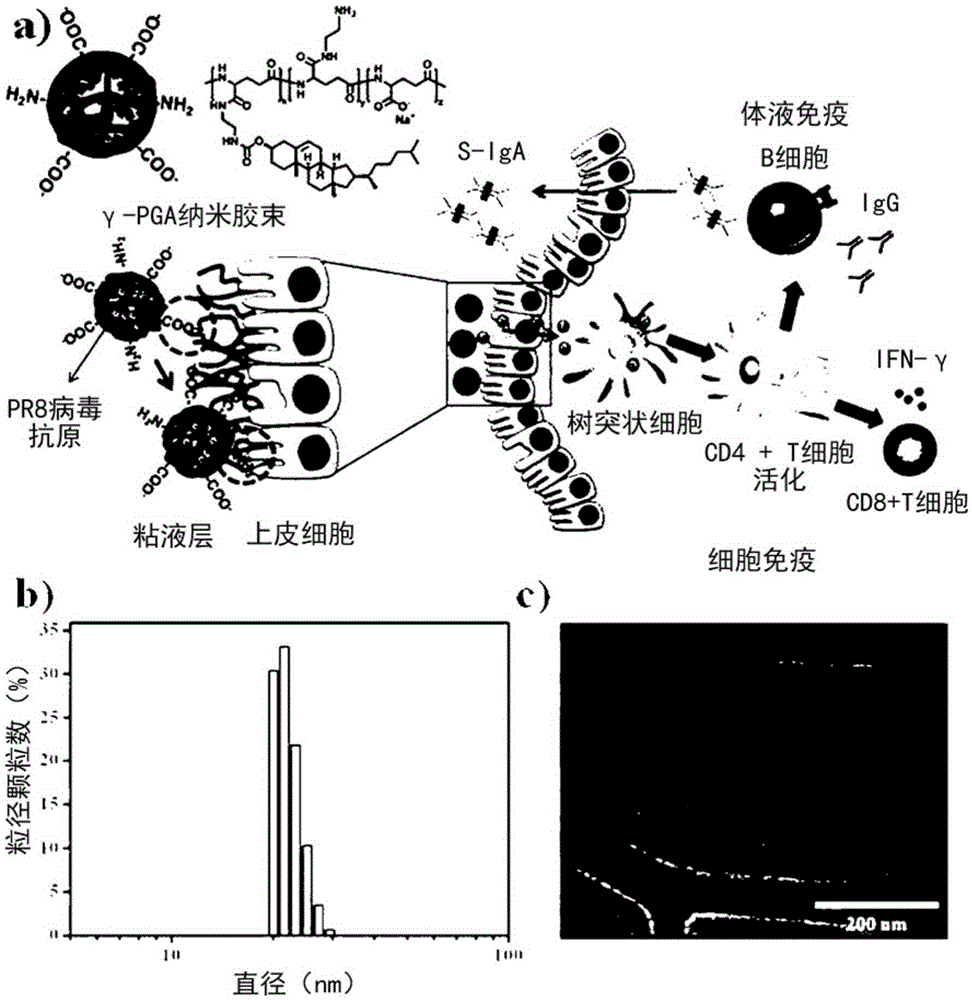
[0056] Poly-γ-glutamic acid-cholesterol nanomicelles were dispersed in distilled water at 1 mg / mL, and measured with DLS (Dynamic Light Scattering (Dynamic Light Scattering), Otsuga, Japan), the results were as follows figure 1 The diameter was confirmed to be 22.1±2.0nm as in (b), and analyzed by Cryo-TEM, the results are as follows figure 1 Spherical poly-γ-glutamic acid-cholesterol nanomicelles were confirmed as in (c). In addition, as a result of NMR analysis, cholesterol was 1.7 mol%, and elemental analysis confirmed that about 28.1 mol% of amino groups were substituted in the poly-γ-glutamic acid-cholesterol nanomicelles. In addition, as a result of measurement by the dynamic scattering method, it was confirmed that the surface charge was about 36.43 mV.
Embodiment 3
[0057] OVA encapsulation of poly-γ-glutamic acid-cholesterol nanomicelles
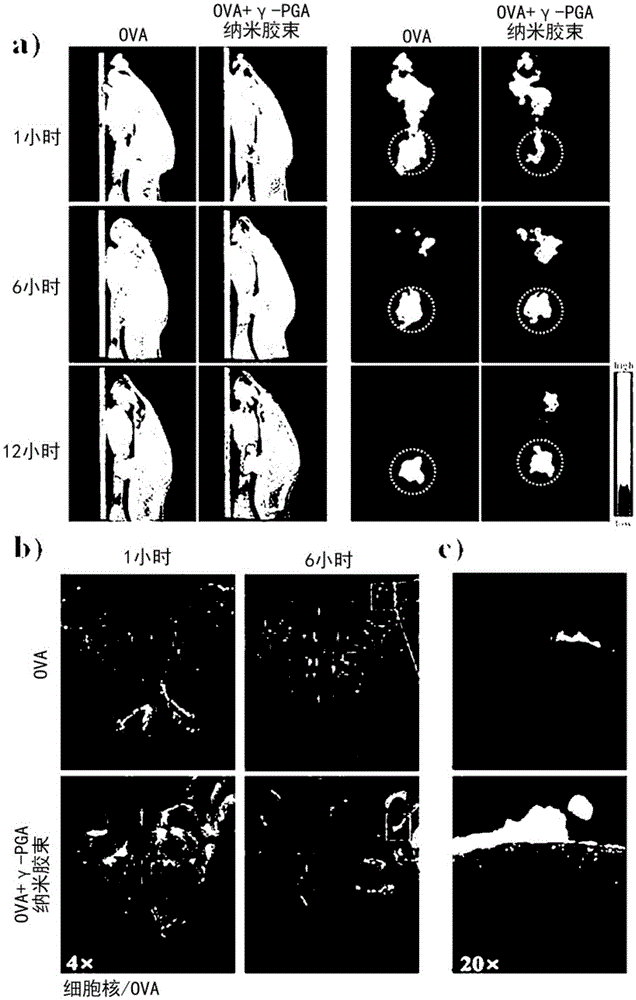
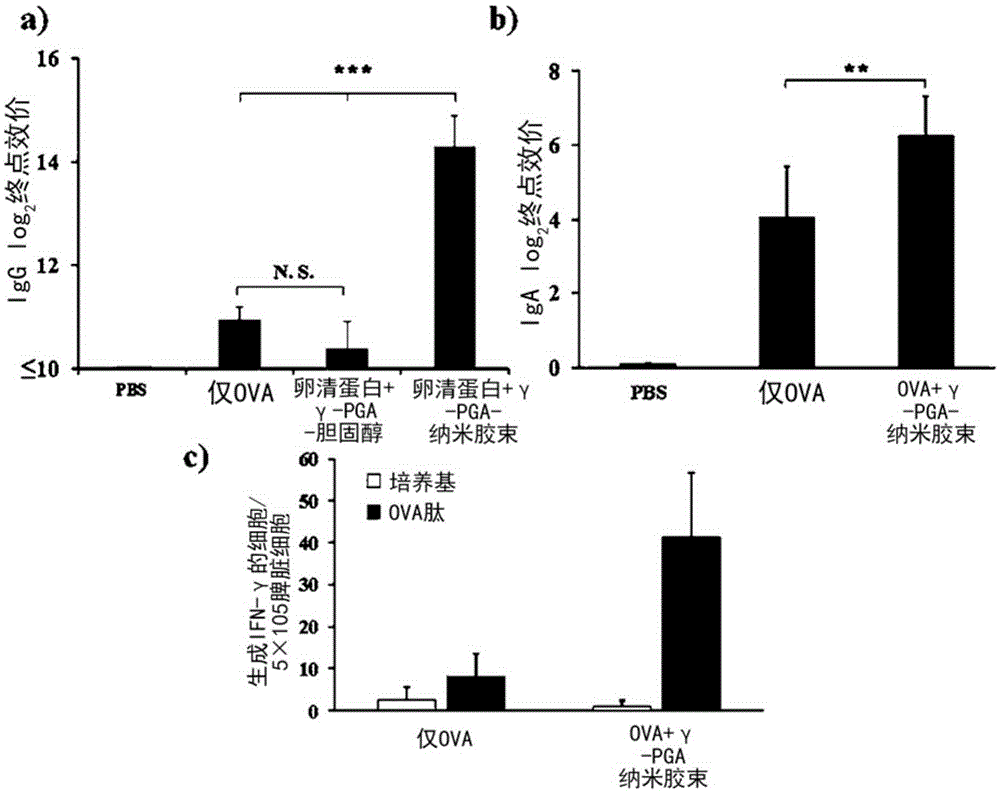
[0058] In order to encapsulate OVA (ovalbumin, Sigma-Aldrich, U.S.) in the poly-gamma-glutamic acid-cholesterol nanomicelle prepared in embodiment 1, the OVA of the nanomicelle of 8mg / mL and 10mg / mL is with mass ratio 5: When mixed at a ratio of 1 and reacted for 1 hour, OVA-encapsulated poly-γ-glutamic acid-cholesterol nanomicelles were prepared. In order to find the proportion of OVA that is 100% enclosed and free, 8 mg / mL poly-γ-glutamic acid-cholesterol nanomicelles and 10 mg / mL LOVA were reacted in a ratio of 2:1 to 9:1 in terms of mass ratio, As a result of experimenting with a polyacrylamide gel (SDS-free gel), it was confirmed that 100% was encapsulated at a ratio of 5:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com