Method using 1,4-dihydroxy-9,10-anthraquinone thiosemicarbazone compound as fluorescent probe to detect copper ions
A technology of anthraquinone amino reduction and fluorescent probe, which is applied in the field of optical analysis and detection, and can solve problems such as increasing immune function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Optimization of experimental conditions
[0023] 1. Selection of Probe Molecular Concentration
[0024] Increasing the concentration of probe molecules can increase the absorbance and fluorescence intensity, which is beneficial to improve the selectivity of metal ions and the linear range of the standard curve. However, when the concentration is too high, the fluorescence intensity of the blank will also increase with the increase of the probe molecule concentration. If the concentration is too low, it is difficult to detect the fluorescence absorption peak after adding ion quenching. Considering the sensitivity and linear range of the method, The concentration of the probe was chosen to be 1.0 × 10 -5 mol / L.
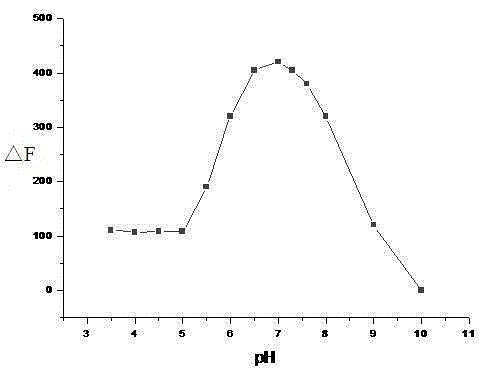
[0025] 2. The choice of buffer solution
[0026] Add 1 mL of phosphate buffer solution to a 10 mL colorimetric tube, and then add 0.01 mL of a molar concentration of 1.0 × 10 -3 mol / L DMF-H of 1,4-dihydroxy-9,10-anthraquinone thiosemicarbazide 2 O solution, ...
Embodiment 2
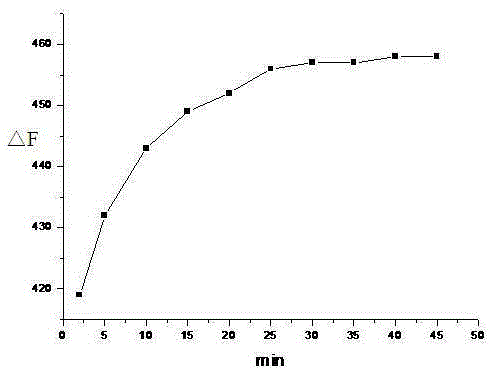
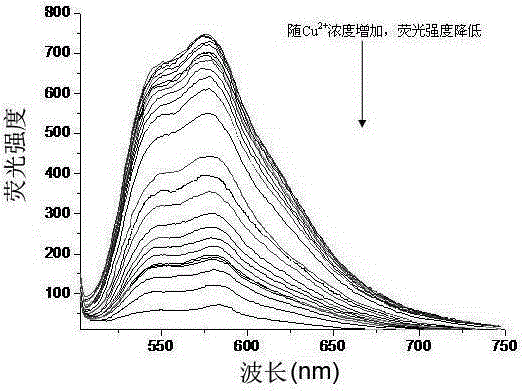
[0033] Add 1 mL of phosphate buffer solution to multiple 10 mL colorimetric tubes, and then add 0.01 mL of 1.0 × 10 molar concentration. -3 mol / L DMF-H of 1,4-dihydroxy-9,10-anthraquinone thiosemicarbazide 2 O (DMF and H 2 The volume ratio of O is 1:99) solution, and then different concentrations of copper ion solutions ([Cu 2+ ](×10 -6 mol / L): 0.3, 0.5, 0.7, 1, 2, 3, 4, 5, 7, 9, 10), dilute to 10 mL with deionized water, shake and mix for 25 minutes, and measure the fluorescence intensity of each solution. The fluorescence intensity measurement conditions are: excitation slit 5nm, emission slit 10nm, excitation wavelength 488nm, emission wavelength 577nm, the solution changes from light red to blue-purple during the measurement process, image 3It is the fluorescence absorption spectrum of copper ion solutions of different concentrations. It can be seen from the figure that the red shift of the fluorescence absorption peak at 488nm appears a new fluorescence absorption pea...
Embodiment 3
[0037] Add 1 mL of phosphate buffer solution to multiple 10 mL colorimetric tubes, and then add 0.01 mL of 1.0 × 10 molar concentration. -3 mol / L DMF-H of 1,4-dihydroxy-9,10-anthraquinone thiosemicarbazide 2 O solution, and then respectively added the molar concentration of 1.0 × 10 -3 mol / L standard solutions of different metal ions (Na + , K + , Ca 2+ , Mg 2+ , Ni 3+ , Mn 2+ , Zn 2+ , Ba 2+ , Fe 3+ , Co 2+ , Cd 2+ , Pb 2+ , Ag 2+ , Hg 2+ different ions), dilute the volume to 10 mL with deionized water, and measure the fluorescence intensity of each solution after shaking (such as Figure 5 ), the results show that when the same concentrations of various metal ions interact with the probe molecule ESN, only Cu 2+ The addition of ions reduced the fluorescence intensity of the mixed system at 577 nm, resulting in fluorescence quenching. When other metal ions interacted with the probe molecules, the fluorescence intensity did not change significantly compared with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com