Binuclear cadmium complex used as nitrobenzene fluorescence recognition sensor and preparation method thereof
A technology for fluorescent recognition and cadmium complexes, applied in cadmium organic compounds, chemical instruments and methods, 2/12 group organic compounds without C-metal bonds, etc., to achieve good selective recognition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthesis of embodiment 1 complex:
[0024] 0.0367g (0.075mmol) 4'-(4-(3,5-dicarboxyphenoxy)phenyl-4,2':6',4"-terpyridine, 0.0215g (0.075mmol) 4,4 '-bis-(imidazolyl)biphenyl, 0.0322g (0.075mmol) Cd(ClO 4 ) 2 ·6H 2 O was dissolved in a mixed solvent of 8mL N,N-dimethylformamide and distilled water in a ratio of 1:1:1 (mass ratio), and the pH value of the mixed solution was adjusted to 5.7 at room temperature, and stirred for 30 minutes. Transfer to the polytetrafluoroethylene liner of a 25mL hydrothermal reaction kettle, react at 150°C for 72 hours, cool to room temperature naturally, and wash the product twice with distilled water (1mL / time) to obtain a light yellow block Crystalline, based on Cd(ClO 4 ) 2 ·6H 2 The calculated yield of O was 78.33%.
Embodiment 2
[0025] Example 2 Structural characterization of complexes:
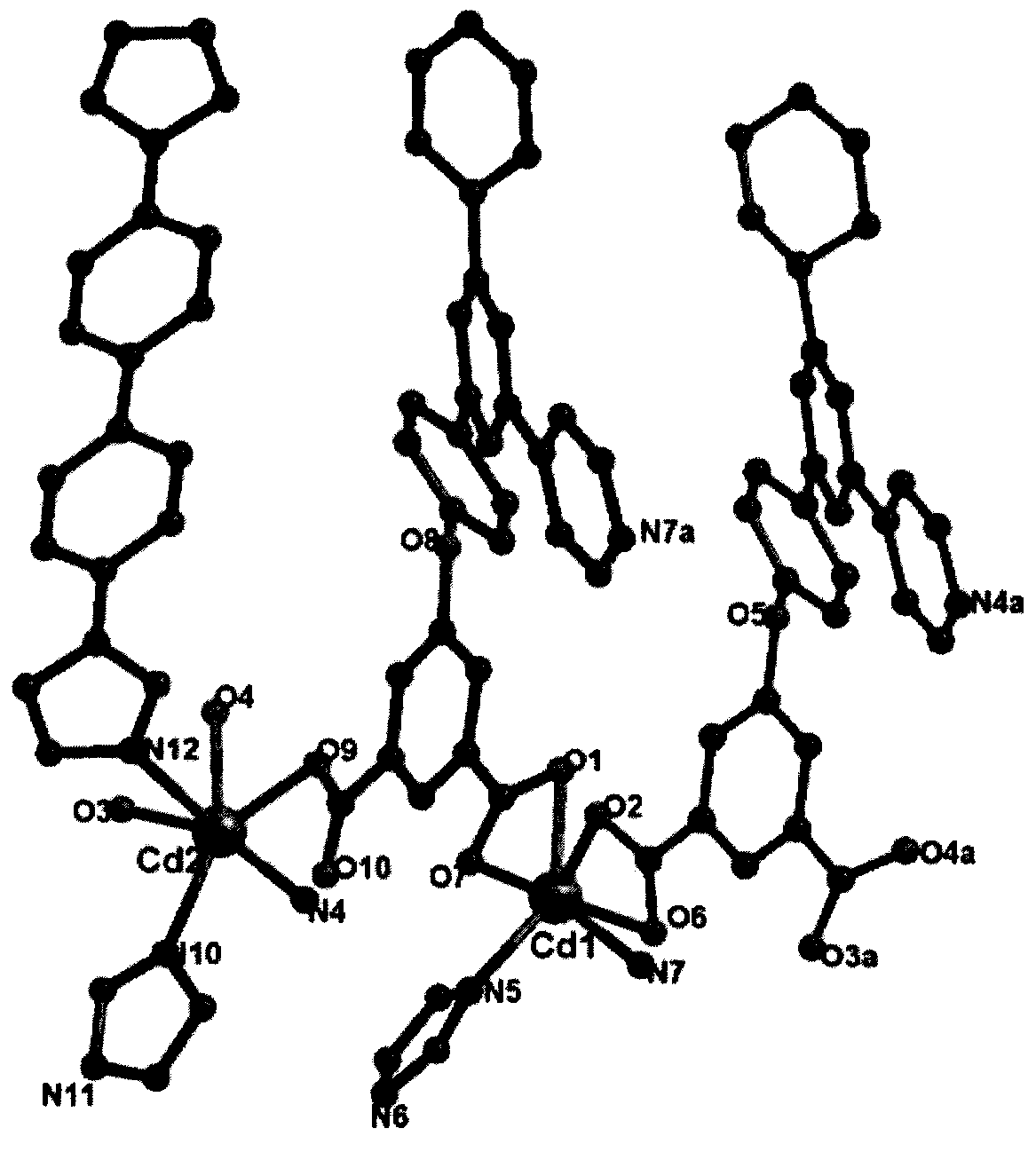
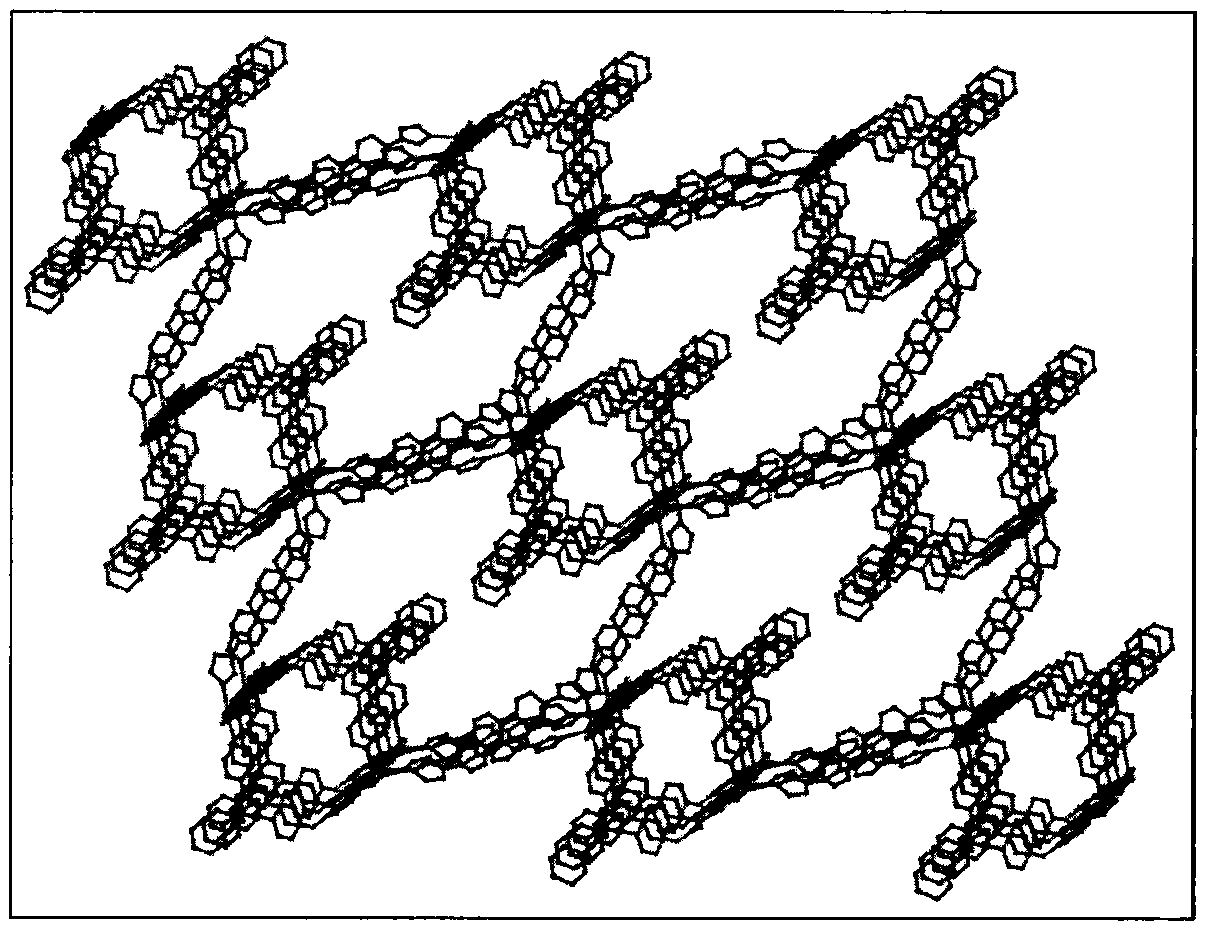
[0026] The crystal structure adopts Bruker Smart CCD X-ray single crystal diffractometer at 296(2)K, and the selected size is 0.34×0.26×0.22mm 3 The bulk crystals, using graphite monochromatized MoKα rays (λ=0.07107nm) as the incident radiation source, collected diffraction points in the way of ω / 2θ scanning, the unit cell parameters were refined by the least square method, and the collected data were collected using the SADABS program The obtained data were corrected for absorption. The structure of the complex was solved by the direct method, the coordinates of non-hydrogen atoms and the anisotropy temperature factor were refined by the full-matrix least-squares method, and all calculations were completed by the SHELXTL program. The detailed crystallographic parameters are listed in Table 1. Binuclear cadmium complex [Cd 2 (DTP) 2 (BIBP) 1.5 ] crystal structure and three-dimensional pore structure such as fig...
Embodiment 3
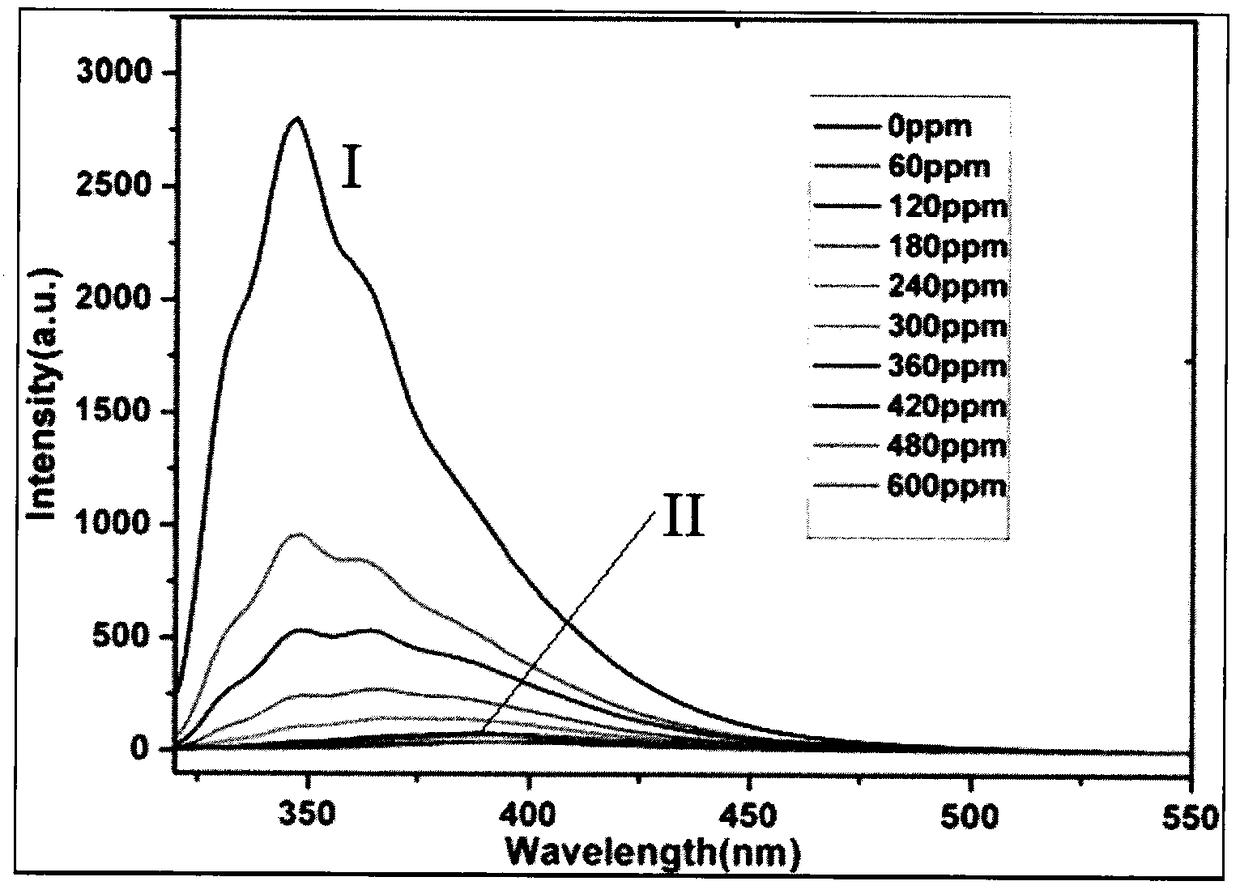
[0029] Example 3 Fluorescent properties of complexes:
[0030] The fluorescence spectrum of the binuclear cadmium complex in N,N-dimethylacetamide (DMA) solution was measured at room temperature by F-7000FL fluorescence spectrometer. The excitation wavelength was 305nm and the emission peak was at 346nm. Compared with the dinuclear cadmium complex blank sample (without adding any organic compound to be detected), when the concentration of nitrobenzene (Nitrobenzene, NB) varies between 0ppm and 600ppm, the emission peak position of the complex does not shift, However, the intensity of its emission peak weakens significantly with the increase of the concentration of nitrobenzene. When the concentration of nitrobenzene is 300ppm, the quenching efficiency of the fluorescence emission peak of the complex can reach 99.8%, the fluorescence intensity of the complex is reduced from 2789a.u. to 36a.u., and the peak intensity is reduced to the corresponding peak of the blank sample abou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com