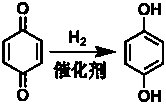
Method for preparing hydroquinone by p-benzoquinone
A technology of hydroquinone and p-benzoquinone, which is applied in the field of preparation of hydroquinone, can solve problems such as easy poisoning and deactivation, increased processing costs, and rapid deactivation, and achieve important application value, reduced use cost, and high purity less demanding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
[0017] Add self-made p-benzoquinone (1.2g, 11.1mmol), metal catalyst (0.12g) and THF (44.0g, 50mL) into a 250mL reactor, mix well, close the reactor and fill it with 40atm hydrogen, stir and heat to 70 o C, after reacting for 24 hours, cool to room temperature, and take out the mixture for analysis after degassing.
[0018] project catalyst Hydroquinone yield (%) 1. 1%Pd / C 94 2. 3%Pd / C 97 3. 5%PtFe / C 94 4. 5%IrFe / C 86 5. 5%PdNi / C 96 6. 5%RuNi / C 60 7. Raney Nickel 95 8. 10%Ni / C 92
Embodiment 9-13
[0020] The experimental procedure is the same as that of Example 8, except that the THF in the third experimental step is replaced with other solvents, and the reaction results are shown in the table below.
[0021] project Solvent (mL) Yield (%) 1. Dichloromethane (50) 98 2. DMF (50) 98 3. Ethanol (50) 82 4. Dioxane (50) 80 5. Ethylene glycol dimethyl ether (50) 84
Embodiment 14-16
[0023] The experimental procedure is the same as in Example 8, only the reaction temperature in the third experimental step is changed, and the influence on the reaction result is shown in the table below.
[0024] project temperature( o c) Yield (%) 1. 40 52 2. 80 96 3. 150 90
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com