Quinic acid lactone derivative preparation method
A technology of ethyl acetate and isopropyl acetate, applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of low solubility, large demand for compound 1, and low reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
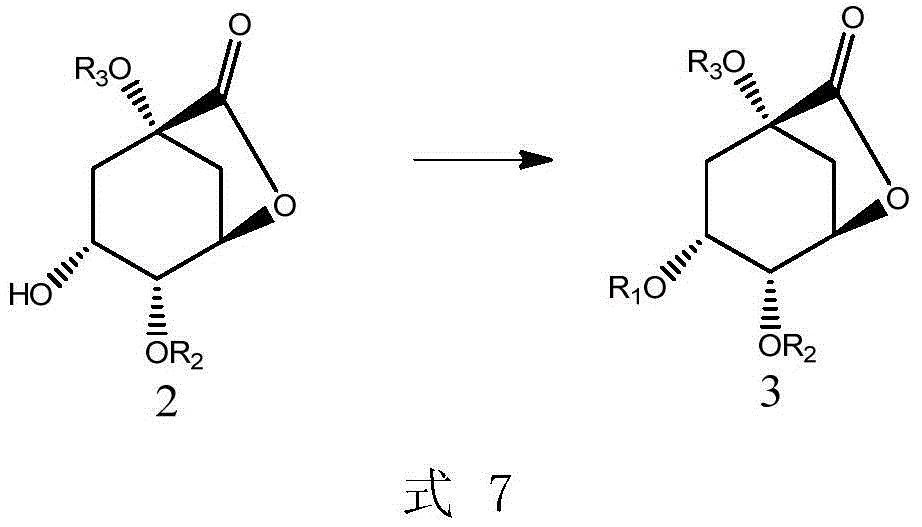
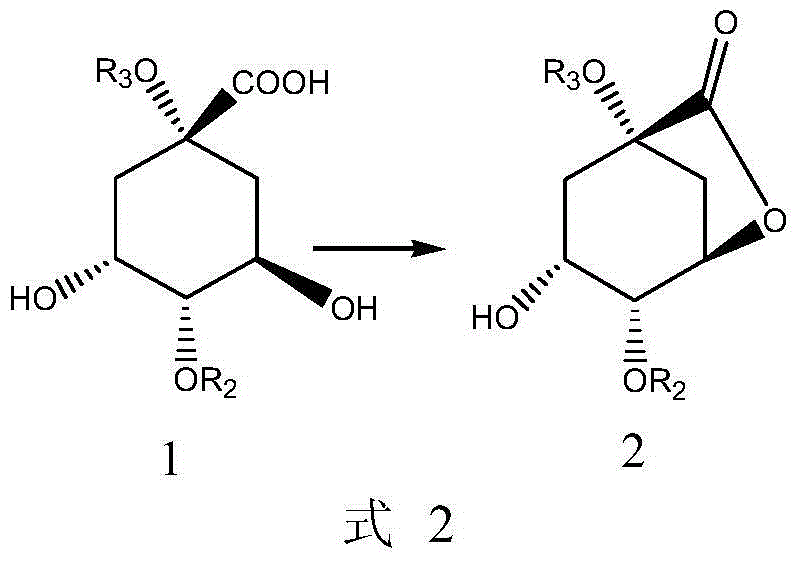
[0061] The present invention provides a preparation method of compound 2 shown in the following formula, characterized in that it comprises steps:
[0062]
[0063] (i) in an organic solvent, react with compound 1 to obtain compound 2;
[0064] Wherein, the organic solvent is selected from the group consisting of n-hexane, cyclohexane, DMF, ethyl acetate, isopropyl acetate, dichloroethane, or combinations thereof.
[0065] In the formula, R 2 , R 3 Each is independently selected from the following group: H, C1-C6 alkyl, or silicon protecting group; preferably, the silicon protecting group is selected from the following group: TBDMS-OTf, TIPS-OTf, or TES-OTf .
[0066] In another preferred example, R 2 for H, R 3 is H or alkyl.
[0067] In another preferred example, the organic solvent is a mixed solution of DMF and cyclohexane.
[0068] Said reaction can optionally be carried out in the presence of a suitable dehydrating condensation agent. In a preferred embodiment...
Embodiment 1
[0118]
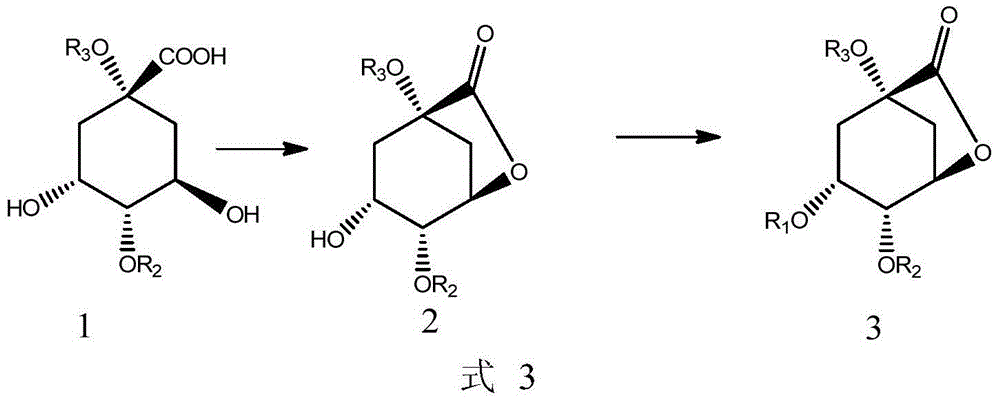
[0119] Using compound 1 as a starting material, compound 1 was added into n-hexane, and the catalyst p-toluenesulfonic acid was added, and reacted at 90° C. for 18 hours. The reaction is shown in formula 12, and the synthesis from compound 2 to compound 3 is carried out with reference to WO2010 / 120698A1. The two-step yield was 25%. where R 1 for TBDMS, R 2 for H, R 3 for H.
Embodiment 2
[0121]
[0122] Using compound 1 as a starting material, compound 1 was added to a mixture of DMF and n-hexane, and a catalyst p-toluenesulfonic acid was added, and reacted at 90° C. for 18 hours. The reaction is shown in formula 13, and the synthesis from compound 2 to compound 3 is carried out according to the method disclosed in PCT application WO2010 / 120698A1, and the two-step yield is 48%. where R 1 for TBDMS, R 2 for H, R 3 for H.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com