Anti-human Delta like 4 monoclonal antibody and application thereof
A monoclonal antibody, DNA molecule technology, applied in the direction of antibody, application, anti-animal/human immunoglobulin, etc., can solve the problems of providing blood flow, inability to tumor tissue, lack of fusion of blood vessels, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: Preparation of anti-D114 monoclonal antibody:
[0020] (1) Immunized mice
[0021] The monoclonal antibody was screened and prepared using recombinant human Dll4 (purchased from Beijing Yiqiao Shenzhou) as the immunogen, dissolved the recombinant antigen in PBS, mixed with quickantibody adjuvant in equal volume, and immunized 6-week-old BALB / c mice. The immunization process Follow the instructions of QuickAntibody. After 21 days, a dose of booster immunization will be given in the same way. On the 35th day, the antigen pulse immunization was carried out according to the conventional method. One week after each immunization, 500 μl of blood was collected from the orbit by the capillary blood collection method. After resting at room temperature for 1 hour, the whole blood was centrifuged at 4°C and 4000 rpm for 15 minutes, and the supernatant serum was collected, and the mouse titer was determined by indirect ELISA. The test results showed that the serum...
Embodiment 2
[0026] Example 2: Amplification of Anti-D114 Monoclonal Antibody Variable Region Gene
[0027] Collect 10 each 7 Total RNA was extracted from four kinds of hybridoma cells in the logarithmic growth phase with an RNA extraction kit, dissolved in 20-50 μl RNase-free water, and stored at -70°C. The first-strand cDNA was synthesized by reverse transcription using total RNA as a template.
[0028] Primers for amplifying the 5′ end of the heavy chain variable region:
[0029] 1. cttccggaattcSARGTNMAGCTGSAGSAGTC
[0030] 2. cttccggaattcSARGTNMAGCTGSAGSAGTCWGG
[0031] Primers for amplifying the 3′ end of the heavy chain variable region:
[0032] ggaagatctCTTGACCAGGCATCCTAGAGTCA
[0033] Primers for amplifying the 5′ end of the Kappa light chain variable region:
[0034] gggagctcGAYATTGTGMTSACMCARWCTMCA
[0035] Primers for amplifying the 3′ end of the Kappa light chain variable region:
[0036] ggtgcatgcGGATACAGTTGGTGCAGCATC
[0037] Among the above primers: R=A, G; Y=C, T; ...
Embodiment 3
[0039] Embodiment 3: Preparation of anti-Dll4 monoclonal antibody
[0040] One week in advance, mice were injected intraperitoneally with 0.5ml paraffin oil. One week after sensitization, the vigorously growing hybridoma cells were centrifuged to discard the medium, resuspended in PBS, and injected intraperitoneally for 10 6 Four kinds of anti-Dll4 hybridoma cells in logarithmic growth phase were injected into the peritoneal cavity of mice. One week later, the ascites of the mice were collected separately.
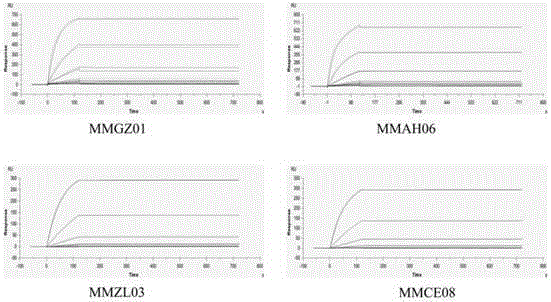
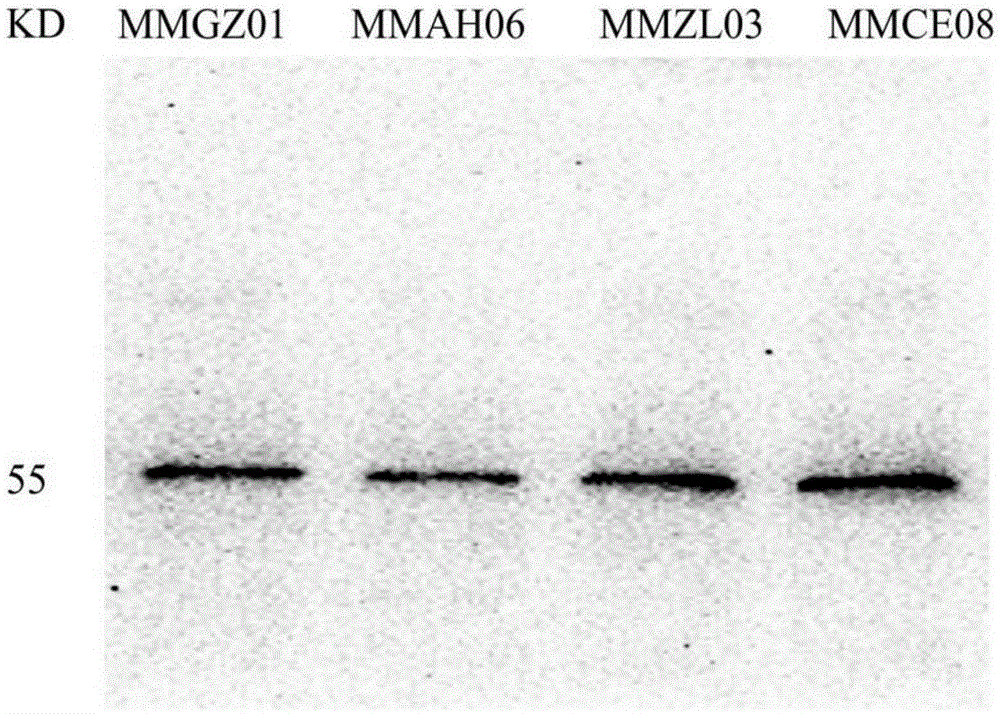
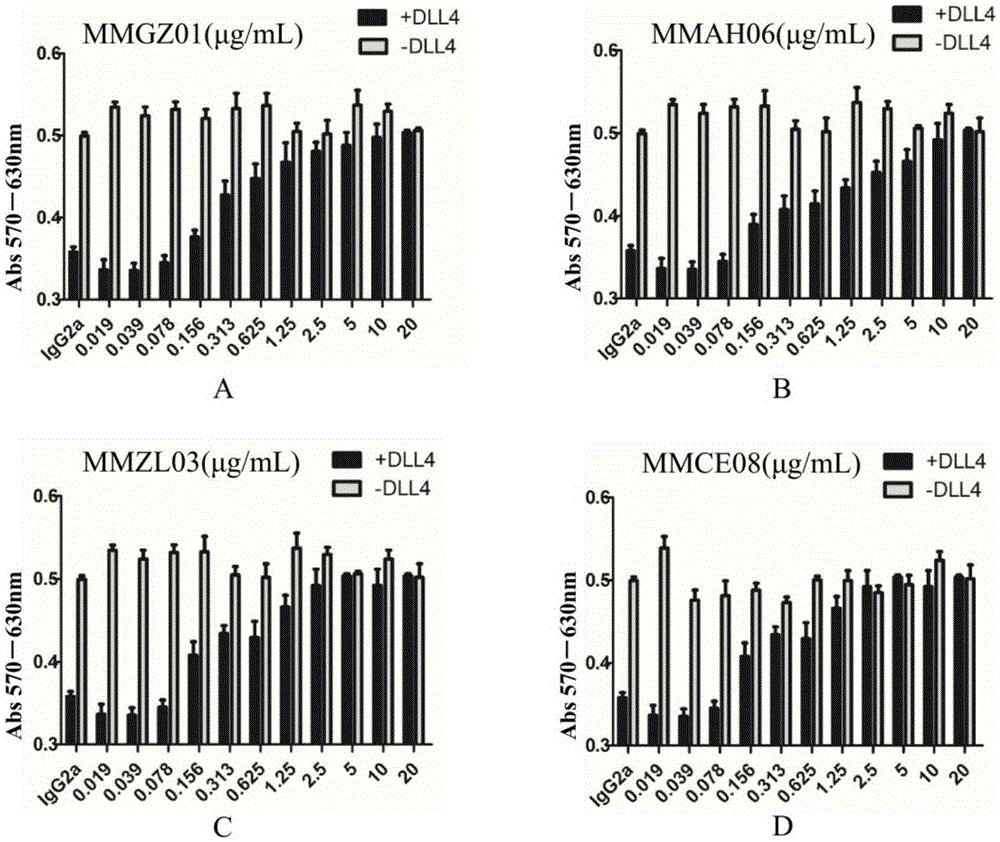
[0041]Centrifuge at 5000g at 4°C for 20min to remove cells and other precipitated substances in the ascites, and then filter with a 0.22μm membrane filter. Purification was carried out according to the manual of Hi-TrapProtein A column of GE Company. After purification, 4 strains of anti-Dll4 monoclonal antibodies were identified by western blotting, and named MMGZ01, MMAH06, MMZL03 and MMCE08 respectively. The results are as follows: figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com