Ibuprofen-codeine phosphate compounding preparation and preparation method therefor
A compound preparation, codeine phosphate technology, applied in the directions of anti-inflammatory agents, pill delivery, pharmaceutical formulations, etc., can solve the problems of poor sustained release effect and large differences in drug release, and achieve good sustained release effect and little mutual influence. , slow-release effect and the effect of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] The present invention also provides a preparation method of the ibuprofen sustained-release pellets described in the above technical scheme, comprising the following steps:
[0070] a) Granulating after mixing codeine phosphate, microcrystalline cellulose, filler and binder, to obtain codeine phosphate immediate-release pellets;
[0071] b) granulating after mixing ibuprofen, disintegrating agent, filler and binding agent to obtain drug-containing pills; the disintegrating agent is microcrystalline cellulose;
[0072] c) coating the drug-containing pill with a sustained-release material to obtain ibuprofen sustained-release pellets; the sustained-release material is polymethacrylate or ethylcellulose;
[0073] d) mixing the ibuprofen sustained-release pellets and codeine phosphate immediate-release pellets to obtain a profen-codeine compound preparation.
[0074] In the present invention, codeine phosphate, microcrystalline cellulose, filler and binder are mixed and gr...
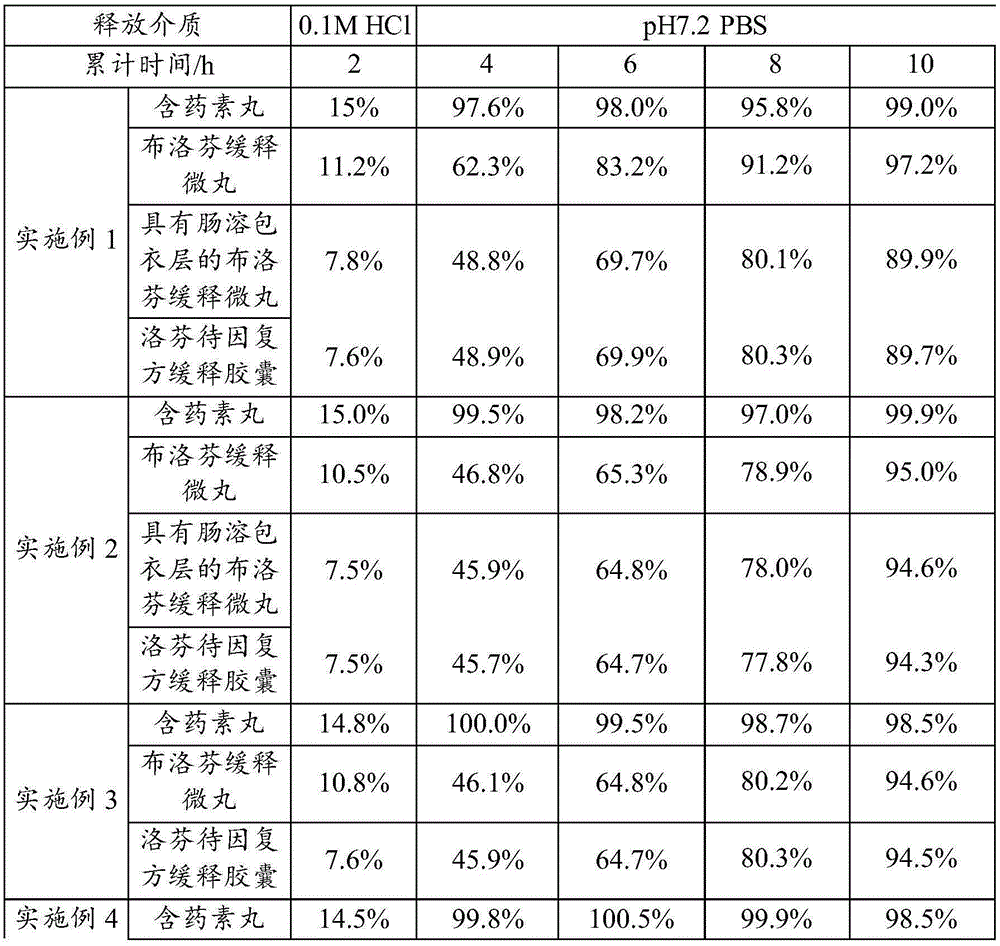
Embodiment 1
[0120] (1) 13g codeine phosphate crude drug, 60gMCCPH302, and 60g starch are stirred and evenly mixed in the HLSH2-6 type wet mixing granulator, and the chassis rotating speed of stirring is 600rpm, and the side disk rotating speed is 1200rpm to obtain the mixed powder; then Keep stirring condition constant, add 71g mass fraction in above-mentioned mixed powder and be 10% starch slurry, obtain soft material; Then above-mentioned soft material is moved to E50 type extruder, extruding under the condition that extrusion speed is 30rpm After 2 minutes, the strips were obtained; then the above-mentioned strips were spheronized in the s-250 type spheronizer for 3 minutes, and the rotating speed of the spheronization was 1200rpm to obtain vegetarian pills; Dry for 12 hours at a drying temperature of 40°C; finally pass through a 18-20 mesh sieve to obtain codeine phosphate immediate-release pellets.
[0121] (2) Stir and mix 200g ibuprofen crude drug, 90gMCCPH302, and 10gLAC in the HL...
Embodiment 2
[0126] (1) Stir 13g of codeine phosphate raw material, 40gMCCPH302, and 80g of calcium hydrogen phosphate in the HLSH2-6 type wet mixing granulator, the chassis rotating speed of stirring is 600rpm, and the side disk rotating speed is 1200rpm to obtain mixed powder Then keep stirring condition constant, add 71g mass fraction in the above-mentioned mixed powder and be 10% PVPK30 aqueous solution, obtain soft material; Then above-mentioned soft material is moved to E50 type extruder, under the condition of 30rpm in extruding speed Extrude for 2 minutes to obtain strips; then spheronize the above-mentioned strips in the s-250 type spheronizer for 3 minutes, and the rotation speed of the spheronization is 1200rpm to obtain vegetarian pills; then put the above-mentioned vegetarian pills in CS101-2ABN hot air circulation Dry in an oven for 12 hours at a drying temperature of 40°C; finally pass through a 18-20 mesh sieve to obtain codeine phosphate immediate-release pellets.
[0127]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com