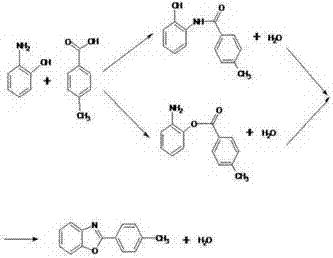
2‑(4‑methylphenyl)‑benzoxazole synthesis process wastewater recycling method
A technology of methyl phenyl and benzoxazole, which is applied in the field of recovery and treatment of p-toluic acid and o-aminophenol, and can solve the problems of unseen recovery and utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0026] Embodiments 1-3 are the recovery and treatment methods of p-toluic acid and o-aminophenol in 2-(4-methylphenyl)-benzoxazole synthesis process wastewater
[0027] specifically:
Embodiment 1
[0029] Take 500ml of condensation process reaction synthesis water collected in the workshop (about 20-30 times the volume of the resin), acidify it to pH 1.5 with dilute hydrochloric acid, stir, the solution turns from dark green to red, and there is yellow precipitation, filter, and recover the yellow color 2.35g of crude p-toluic acid (purity 94.52%, HPLC); the filtrate was adjusted to pH 6 with 20% sodium sulfite solution, and the color of the solution changed from red to dark yellow, and then continued to adjust the pH with 20% sodium hydroxide solution When the value reaches 8.5-9, use adsorption resin (CD101 resin) to pass through the column at a speed of 30-40ml / h for adsorption. 50ml hydrochloric acid solution for elution and analysis, the elution speed is the same as that of the column, the eluate is neutralized with 40% sodium hydroxide and the pH value is adjusted to 8.5-9, and concentrated under reduced pressure to 1 / 3-1 / 2 of the original volume , the temperature ...
Embodiment 2
[0031] Take 500ml of condensation process reaction synthesis water collected in the workshop (about 20-30 times the volume of the resin), acidify it to pH 1.5 with dilute hydrochloric acid, stir, the solution turns from dark green to red, and there is yellow precipitation, filter, and recover the yellow color 2.33g of crude p-toluic acid (purity 94.50%, HPLC); the filtrate was adjusted to pH 6 with 30% sodium hydrosulfide solution, the color of the solution changed from red to dark yellow, and then continued with 20% sodium hydroxide solution Adjust the pH value to 8.5-9, and use the adsorption resin (CD101 resin) to pass through the column at a speed of 30-40ml / h for adsorption. 50ml of 15% hydrochloric acid solution for elution, the elution speed is the same as that of the column, the eluate is neutralized by 40% sodium hydroxide and the pH value is adjusted to 8.5-9, and concentrated under reduced pressure to 1 / 3-1 / 3 of the original volume 2. Lower the temperature to precip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com