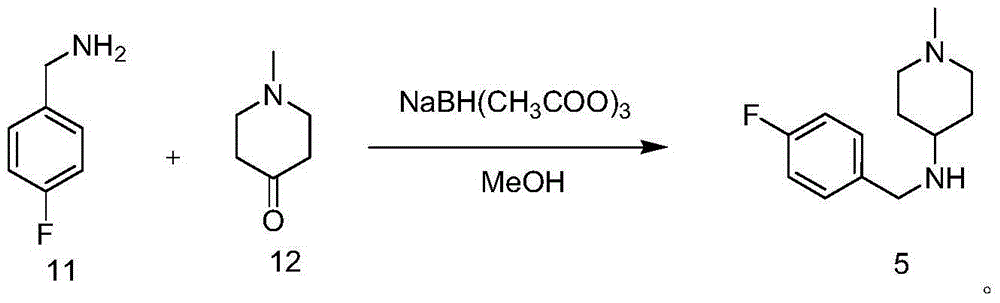
Intermediate of pimavanserin and similar compound thereof, and preparation method thereof, and method for preparing pimavanserin and similar compound thereof
A technology of pimavanserin and compounds, which is applied to the preparation of organic compounds, chemical instruments and methods, and the preparation of carbamic acid derivatives, etc., can solve problems such as potential safety hazards, and achieve low price, simple operation, and easy separation and purification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
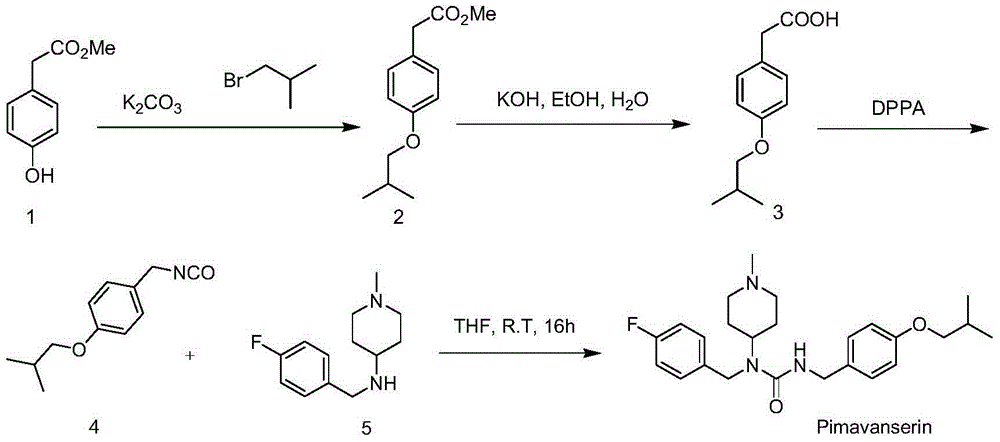
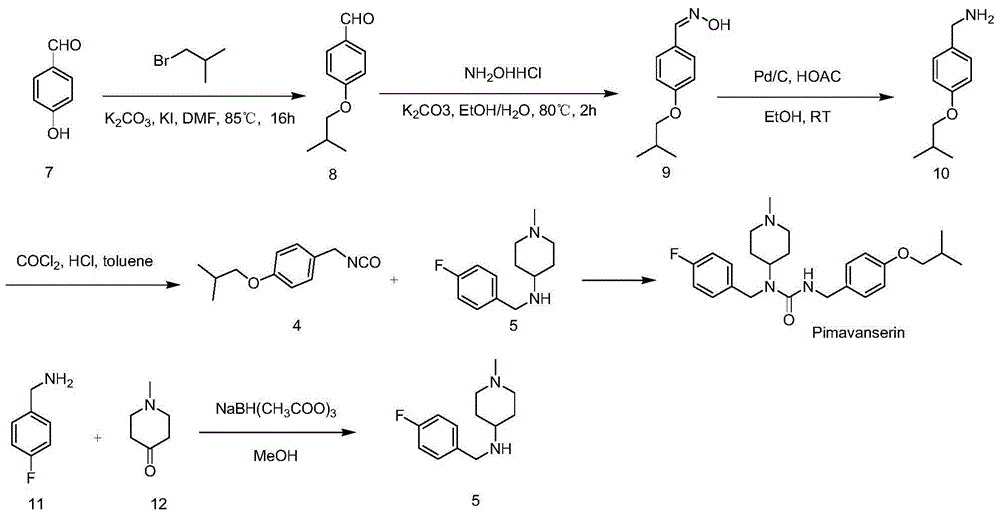
[0056] Embodiment 1 prepares compound 8 by compound 7
[0057]
[0058] Add compound 7 (10.0g, 81.9mmol), anhydrous potassium carbonate (22.6g, 163.8mmol), potassium iodide (1.4g, 8.2mmol) and 50mL DMF into a 250mL three-necked flask, heat to 85°C, and slowly add bromine to the reaction system Substituted isobutane (22.4g, 163.8mmol), after addition, the system was reacted at 85°C for 3h, then continued to slowly add bromoisobutane (11.2g, 81.9mmol) to the system, and the system was continued at 85°C Reaction 3h. The reaction was stopped, the system was cooled to room temperature, filtered, and the filter cake was washed twice with 100 mL of ethyl acetate. Pour the filtrate into 250mL water, separate the organic phase, extract the water phase with 100mL ethyl acetate three times, combine the organic phases, wash once with 150mL saturated NaCl aqueous solution, dry over anhydrous sodium sulfate, filter after drying, and spin the filtrate to obtain 14.0 g of compound 8, li...
Embodiment 2
[0060] Embodiment 2 prepares compound 9 by compound 8
[0061]
[0062] Add compound 8 (10.0g, 56.1mmol), 250mL ethanol, K 2 CO 3 (38.7g, 280.5mmol); Hydroxylamine hydrochloride (19.5g, 280.5mmol) was dissolved in 15mL of water, and then added to the system, after addition, the system was heated and refluxed for 2h, the reaction was stopped, cooled to room temperature, and the system was filtered. The filtrate was rotary evaporated to 100 mL, poured into 250 mL of water, and extracted three times with 100 mL of ethyl acetate. The organic phases were combined, washed once with 150 mL of saturated NaCl aqueous solution, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain a crude product, which was purified by column chromatography (developer polarity: petroleum ether / ethyl acetate=20 / 1) 8.1 g of compound 9 was obtained as a white solid with a yield of 75%.
[0063] The NMR data of compound 9 are as follows: 1 HNMR(400MHz,DMSO)δ10.9...
Embodiment 3
[0064] Embodiment 3 prepares compound 10 by compound 9
[0065]
[0066] Compound 9 (4.0 g, 20.7 mmol), 60 mL of ethanol, acetic acid (2.0 g, 33.1 mmol) and Pd / C (1.2 g, Pd content 10%) were added to a 250 mL three-necked flask. After the addition, the system was catalytically hydrogenated at room temperature and atmospheric pressure. After reacting for 12 hours, filter, wash the filter cake with 30 mL of ethanol, combine the filtrate, and concentrate the filtrate to obtain an oil. Add 50 mL of ethyl acetate and 100 mL of petroleum ether to the oily matter and shake well. After standing still for 3 h, a large amount of white solid (the acetate form of compound 10) precipitates. Filter to obtain 2.3 g of white solid, add 20 mL of 30% NaOH aqueous solution, extract three times with ethyl acetate (15 mL × 3), combine the organic phases, wash with 10 mL of water and 10 mL of saturated NaCl solution once, dry over anhydrous sodium sulfate, filter, and the filtrate Concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com