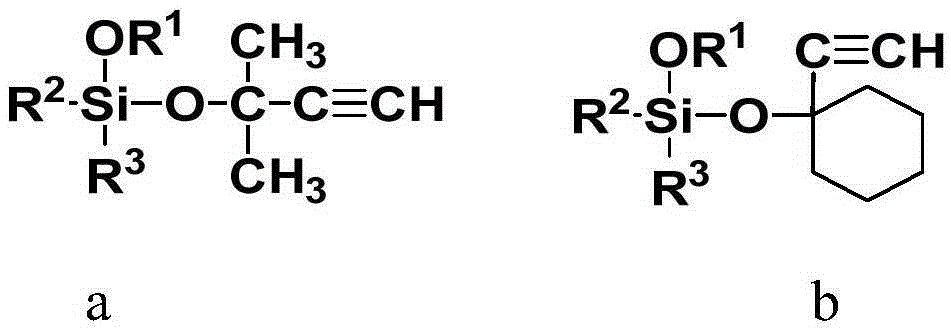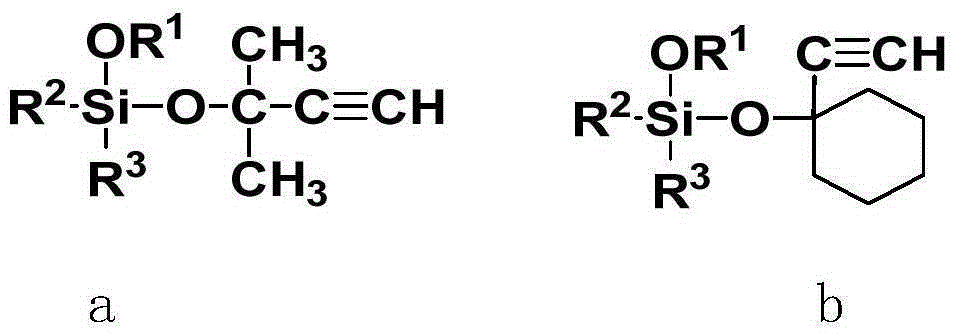Alkoxy silane acetylenic silicon hydrogen addition inhibitor and preparation method thereof
A technology for alkoxysilylation of alkynes and hydrosilylation, which is applied in the field of multifunctional alkoxysilylation of acetylenic hydrosilylation inhibitors and their preparation, and can solve the problem of poor storage stability of glue A, etc. To solve the problem of toxicity, good reproducibility of the reaction, and the effect of reducing volatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0023] Embodiment two, preparation
[0024] In a 250ml three-necked round-bottom flask equipped with a magnet, a reflux condenser, a bubbler and a nitrogen device, add successively, 12.4g (0.1mol) ethynyl cyclohexanol, 30.4g (0.2mol) tetramethoxysilane , 0.1 g butyl titanate. React at a temperature of 60 degrees for 24 hours to obtain a blood red liquid. After cooling, the oil pump reduced pressure distillation to remove low boilers, and then raised the temperature to 90°C to extract the product to obtain 24.0 g of a colorless transparent liquid. 1 H-NMR (CDCl 3 ,300MHz)δ[ppm]:3.60(s,9H,OCH 3 ), 2.53(s,-C≡C-H), 1.95(br,2H), 1.69-1.59(m,5H), 1.58-1.49(m,2H), 1.26(br,1H)
Embodiment 3
[0025] Embodiment three, preparation
[0026] Add 12.6g (0.15mol) methyl butynol, 44.4g (0.3mol) vinyltrimethoxysilane successively in the 250ml three-neck round-bottomed flask equipped with magneton, reflux condenser, bubbler and nitrogen device , 0.15g stannous isooctanoate, react at 90 degrees for 6 hours. After cooling, a pale yellow liquid was obtained. The oil pump decompressed to remove the low boilers, and then heated up to 60°C to extract the product to obtain 29.5 g of a colorless transparent liquid. 1 H-NMR (CDCl 3 ,300MHz)δ[ppm]:6.16-5.88(m,3H,-CH=CH 2 ),3.58(s,6H,OCH 3 ),2.45(s,1H,-C≡C-H),1.59(s,6H,-CH 3 )
Embodiment 4
[0027] Example 4, preparation
[0028] In the 250ml three-neck round-bottomed flask that is equipped with magneton, reflux condenser, bubbler and nitrogen device, successively 14.2g ethynyl cyclohexanol (0.11mol), 33.8g (0.23mol) vinyltrimethoxysilane, 0.12g potassium carbonate, react at 20 degrees for 20 hours. After cooling, potassium carbonate was removed by centrifugal filtration. Oil pump reduced pressure distillation to remove low boilers, and then heated up to 90 degrees to extract the product to obtain 25.9 g of colorless transparent liquid. 1 H-NMR (CDCl 3 ,300MHz)δ[ppm]:6.11-5.89(m,3H,-CH=CH 2 ),3.54(s,9H,OCH 3 ),2.49(s,-C≡C-H),1.89(br,2H),1.68-1.57(m,5H),1.55-1.48(m,2H),1.26(br,1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com