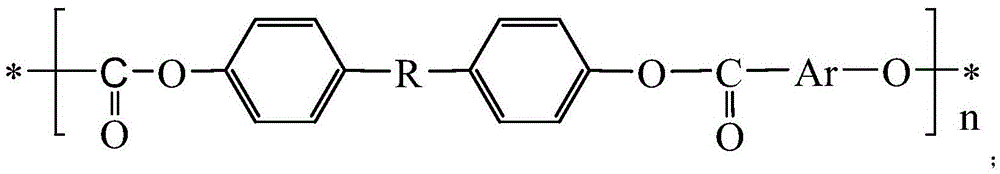
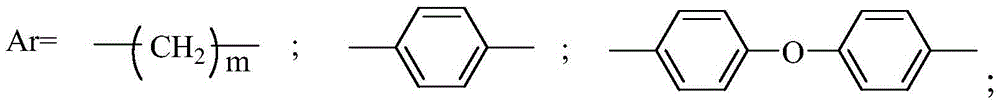
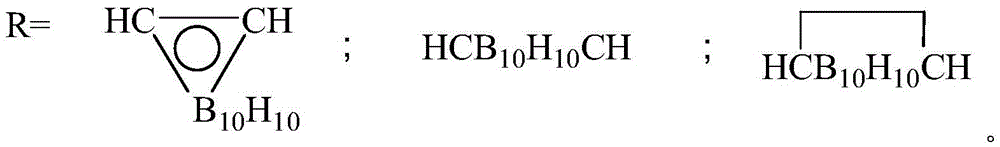Method for synthesizing carborane polyarylester in greenhouse and carborane polyarylester
A technology for carborane polyarylate and room temperature synthesis, which is applied in the field of carborane polyarylate and carborane polyarylate synthesis at room temperature, can solve the problem of high risk, achieve high molecular weight, simple and easy operation, The effect of excellent thermal stability and mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of the carborane polyarylate provided by the invention only needs to be carried out at room temperature, and does not need to be carried out at high temperature like traditional carborane-containing polymers, and is safe and reliable, with high molecular weight and a yield of more than 75%. The process solves the problem of different solubility of the acid chloride phase and phenol relative to the same solvent, resulting in different proportions of raw materials. By controlling the proportion of monomers, the order of feeding, etc., the molecular weight and end group structure of the polymer are regulated; the polymer is dissolved in the organic phase to solve the problem of polymerization The poor solubility of the substance to the system cannot be a problem of increasing the polymer while gradually polymerizing the raw material with a high conversion rate; the operation process is simple and easy. The obtained carborane polyarylate resin has exce...
Embodiment 1
[0049] Synthesis of polyarylate with 4,4-diacid chloride diphenyl ether and 1,2-bis(4-hydroxyphenyl)-o-carborane: in a system equipped with constant pressure funnel, reflux condenser, stirring device, rubber stopper In the 100mL three-necked flask with argon inlet, at room temperature, first add 1,2-bis(4-hydroxyphenyl)-o-carborane (2.05g, 6.25mmol) in the three-necked flask in NaOH aqueous solution ( 0.7M) 15ml, then add benzyltriethylammonium chloride (BTEAC) (0.0125g, 0.055mmol), and slowly add 4,4-diacid chloride diphenyl ether (1.845g, 6.25mmol) dropwise with a constant pressure funnel ) in dichloromethane (37.5ml), stirred slowly, and added dropwise within 30min. Stir vigorously and continue to react at room temperature for 12 hours. The excess solvent was distilled off under reduced pressure, and vacuum-dried at 40 degrees to obtain the white target product: 2.949g, yield 92%; 1100cm in the product -1 The aryl ether peak of R-O-R appears at 2600cm -1 The stretching v...
Embodiment 2
[0051] Synthesis of polyarylate with 4,4-diacid chloride diphenyl ether and 1,7-bis(4-hydroxyphenyl)-m-carborane: in a system equipped with constant pressure funnel, reflux condenser, stirring device, rubber stopper In the 100mL three-necked flask with argon inlet, at room temperature, first add 1,7-bis(4-hydroxyphenyl)-m-carborane (2.05g, 6.25mmol) in the three-necked flask in NaOH aqueous solution ( 0.7M) 8.9ml, then add benzyltriethylammonium chloride (BTEAC) (0.0125g, 0.055mmol), and slowly add 4,4-diacid chloride diphenyl ether (1.845g, 6.25 mmol) in dichloromethane (37.5ml), stirred slowly, and added dropwise within 30min. Stir vigorously and continue to react at room temperature for 12 hours. The excess solvent was distilled off under reduced pressure, and vacuum-dried at 40 degrees to obtain the white target product: 2.55g, yield 89%; 1100cm in the product -1 The aryl ether peak of R-O-R appears at 2600cm -1 The stretching vibration peak of B-H appears at 750cm -1 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Elongation at break | aaaaa | aaaaa |
| Elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com