joint care composition
A composition and dry matter technology, applied in the prevention or treatment of osteoarthritis, the field of composition for the prevention or treatment of osteoarthritis, capable of solving problems related to adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Individual Screening of Compounds
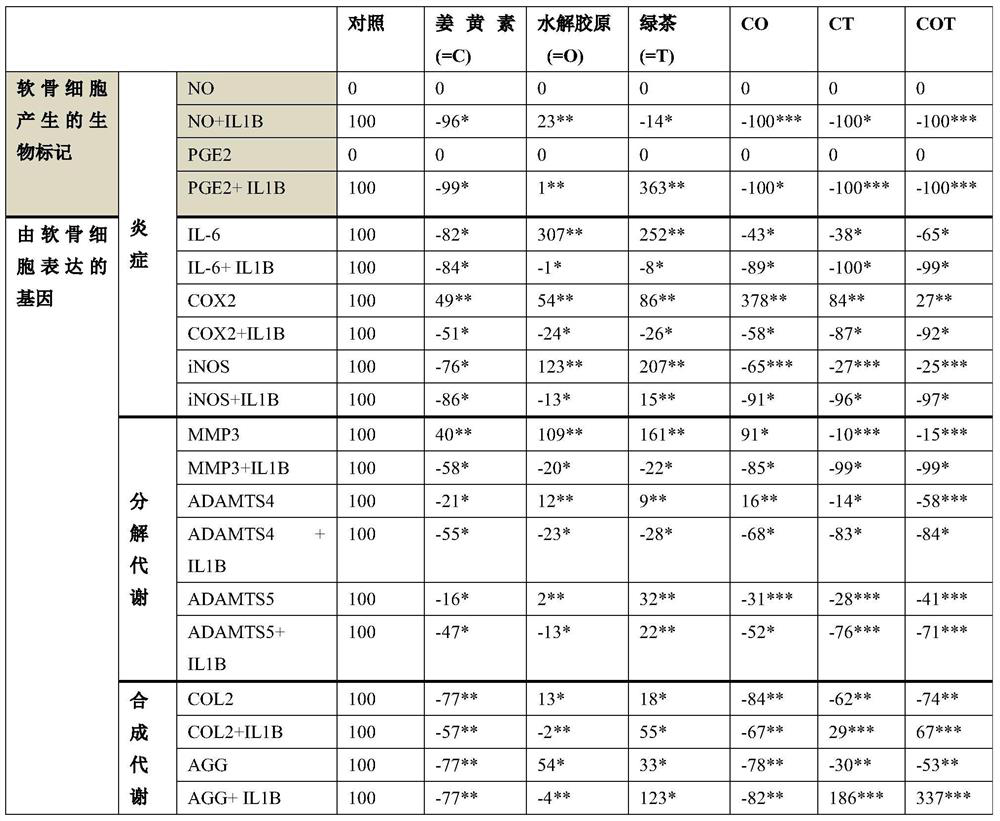
[0068] Experiments were performed to evaluate the effect of several compounds on primary cultures of bovine chondrocytes in which inflammatory and catabolic processes were induced by interleukin-1β to mimic the effects of arthritic chondrocytes.
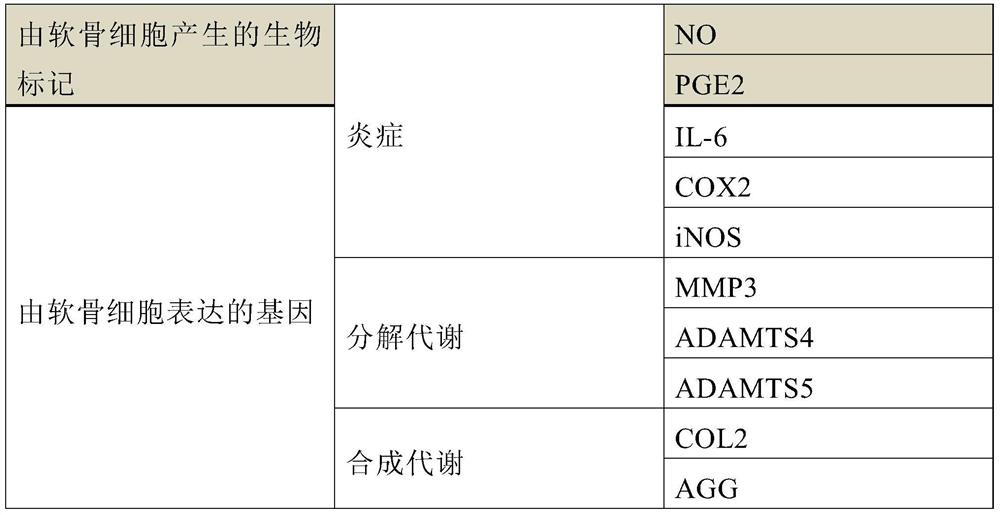
[0069] The table below details the biomarkers determined throughout the experiments to show the effect of the compounds on the three metabolic pathways on chondrocytes.
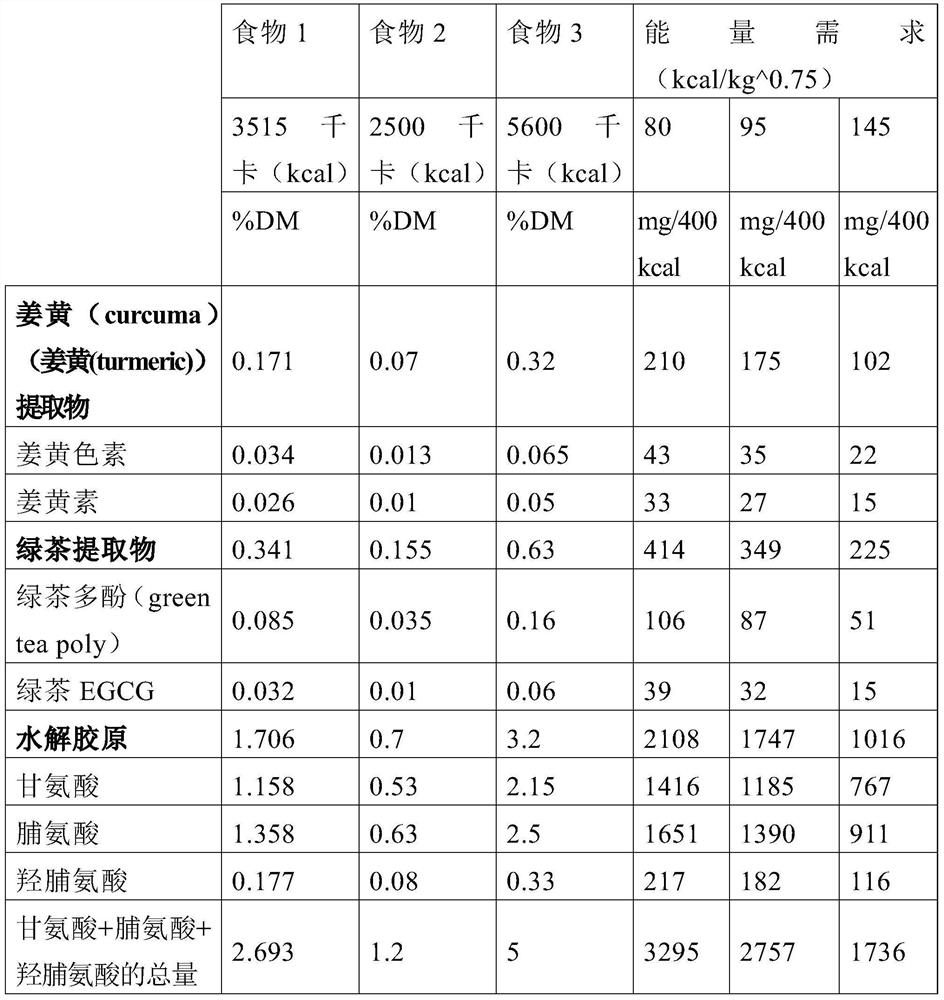
[0070] Table 3: Biomarkers detected
[0071]
[0072] Primary culture of monolayer bovine chondrocytes
[0073]Normal bovine articular cartilage was obtained from the metacarpal-phalangeal joint of shortly after mortem bulls aged 1 to 2 years. Full-depth articular cartilage was excised and immersed in supplemented with N-(2-hydroxyethyl)piperazine-N'-(2-ethanesulfonic acid) (HEPES) 10mM, penicillin (100U / ml) and chain Mycin (0.1 mg / ml) (both from Lonza (Lonza) Verviers, Belgium) Dulbecco's Modified Eagl...
Embodiment 2
[0092] Example 2: Dose Response
[0093] Carry out according to the method for embodiment 1. According to the molecular weight, 4 different concentrations were tested: 0.5 μg / ml, 2.5 μg / ml, 12.5 μg / ml and 62.5 μg / ml to cover the corresponding 10 -5 M concentration range.
[0094] The results showed that, for each compound, the concentration that gave the best effect and gave no toxic effects was 12.5 μg / ml. This is why a concentration of 12.5 μg / ml was used in combination with each other to test the compounds.
Embodiment 3
[0095] Example 3: Specific combinations and synergistic effects of test compounds
[0096] The method of Example 1 was carried out.
[0097] compound supplementation
[0098] When the cells reached confluence, the medium was removed and replaced with fresh medium (supplemented with 1% fetal bovine serum, 10 mM HEPES, 100 U / ml penicillin, 0.1 mg / ml streptomycin, 2 mM glutamine, and 20 μg / ml proline). DMEM without phenol red and containing only 1 g / L glucose) was replaced by fresh medium containing some compounds (12.5 μg / ml each) and with or without recombinant porcine IL-1β (10 -11 M) (RD Systems, Abingdon, UK). Three compounds were tested, turmeric extract (Naturex, Avignon, France), hydrolyzed collagen (Galida, Eberbach, Germany) and green tea extract (Naturex, Avignon Ong, France). Turmeric extract was prepared as a 12.5 mg / ml solution in tetrahydrofuran (Merck, Leuven, Belgium) and further diluted 1000-fold in cell culture medium. Hydrolyzed collagen and green tea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com