Preparation method of lenalidomide
A technology of oxo and dihydroisoindole, which is applied in the field of preparation of lenalidomide (piperidine-2,6-dione), can solve the problems of long reaction time of catalytic reaction reflux, labor protection difficulties, production difficulties, etc. , to achieve the effect of novel process route, less three wastes and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
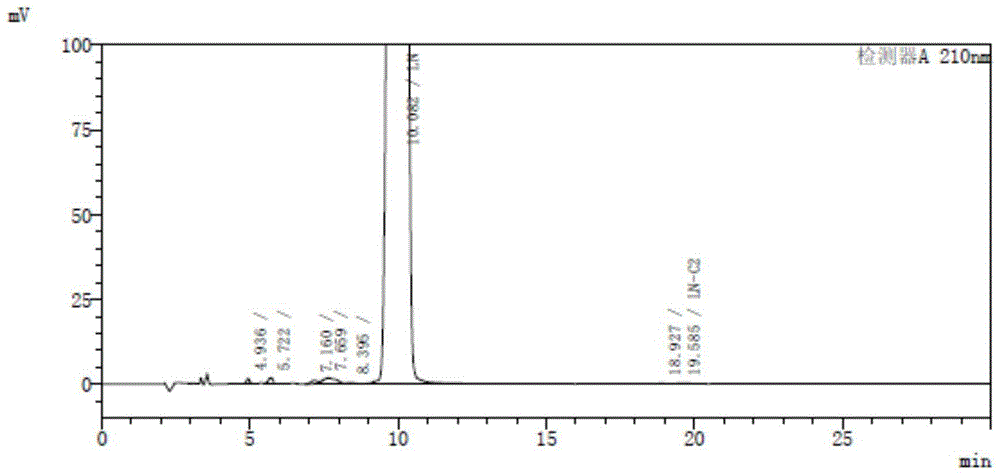
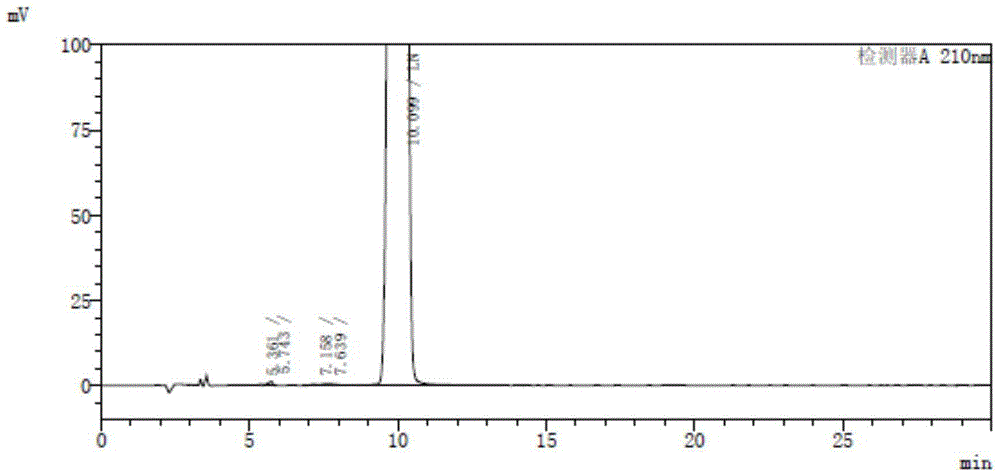
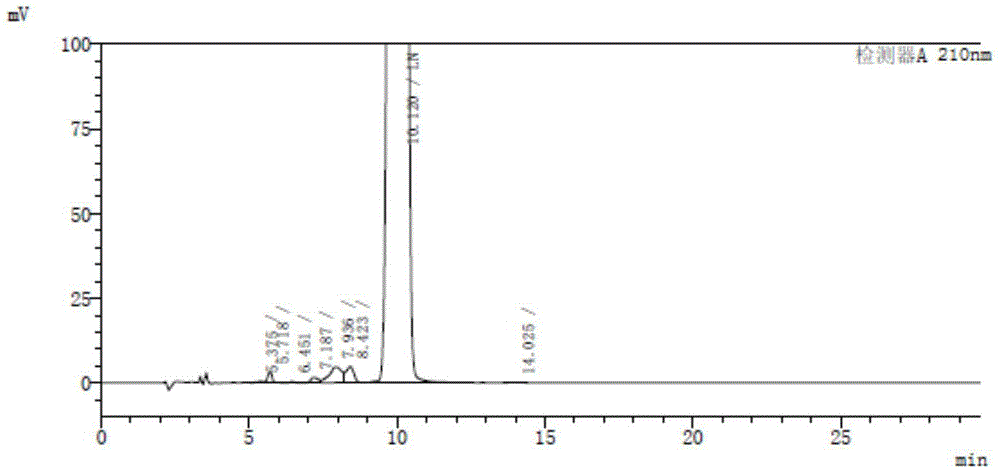
Image
Examples
Embodiment 1
[0048] (1) Preparation of methyl 2-bromomethyl-3-nitrobenzoate
[0049] 15g of methyl 2-methyl-3-nitrobenzoate, 19g of NBS, 1.89g of AIBN, 100mL of water were stirred, the temperature was kept at 80℃, and the reaction was kept at low speed for 1 hour. The organic layer was separated and crystallized in ethanol to obtain The yellow solid was filtered and dried to obtain 18 g of the product with a yield of 90.2%.
[0050] 1 HNMR(500MHz,CDCl3)δ8.12(dd,J=7.5,2.0Hz,1H),8.00(dd,J=7.4,1.9Hz,1H), 7.51(t,J=7.5Hz,1H), 4.46( s, 2H), 3.94 (s, 3H).
[0051] (2) Preparation of dimethyl 3-(7-nitro-1-oxo-1,3-dihydroisoindol-2-yl)glutarate
[0052] 2-bromomethyl-3-nitrobenzoic acid methyl ester 10.03g, D,L-glutamate dimethyl ester 7.78g, sodium carbonate 9.82g, stirred in DMF80mL, reacted at 45℃ for 16 hours, add water 100mL, It was extracted with EA (100 mL*3), concentrated and recrystallized with ethanol to obtain 10.62 g of a yellow solid product with a yield of 86.3%.
[0053] 1 HNMR(500MHz,CDCl3...
Embodiment 2
[0064] (1) Preparation of 2-chloromethyl-3-nitrobenzoic acid methyl ester
[0065] Methyl 2-methyl-3-nitrobenzoate 15g, NCS14.25g, AIBN1.89g, 100mL of water, stir, and keep the temperature at 80℃, keep stirring at low speed for 1 hour, separate the organic layer and crystallize in ethanol A yellow solid was obtained, filtered, and dried to obtain 15.12 g of the product with a yield of 86.2%.
[0066] 1 HNMR(500MHz,CDCl3)δ8.09(dd,J=7.5,2.0Hz,1H), 8.04(dd,J=7.5,2.0Hz,1H), 7.51(t,J=7.5Hz,1H), 4.64( s, 2H), 3.94 (s, 3H).
[0067] (2) Preparation of dimethyl 3-(7-nitro-1-oxo-1,3-dihydroisoindol-2-yl)glutarate
[0068] 8.43g methyl 2-chloromethyl-3-nitrobenzoate, 7.82g D,L-glutamate dimethyl, 9.77g sodium carbonate, stirred in DMF80mL, reacted at 45°C for 16 hours, added water 100mL, It was extracted with EA (100 mL*3), concentrated and recrystallized with ethanol to obtain 9.16 g of a yellow solid product with a yield of 74.4%.
[0069] 1 HNMR(500MHz,CDCl3)δ8.41(dd,J=7.4,1.9Hz,1H), 8.31(d...
Embodiment 3
[0072] (1) Preparation of methyl 2-bromomethyl-3-nitrobenzoate
[0073] 15g of methyl 2-methyl-3-nitrobenzoate, 19g of NBS, 2.11g of azobisisoheptanitrile, 100mL of chloroform, stir, keep stirring and reflux for 8 hours, cool to room temperature, add 200ml of water, stir, separate The organic layer was washed with 20 ml of saturated sodium bicarbonate, the organic layer was separated and dried over anhydrous sodium sulfate, concentrated and crystallized in ethanol to obtain a yellow solid, which was filtered and dried to obtain 13.86 g of the product with a yield of 69.5%.
[0074] (2) Preparation of dimethyl 3-(7-nitro-1-oxo-1,3-dihydroisoindol-2-yl)glutarate
[0075] 2-bromomethyl-3-nitrobenzoic acid methyl ester 10.03g, D,L-glutamate dimethyl ester 7.78g, potassium carbonate 10.63g, stirred in DMAC 80mL, reacted at 45°C for 16 hours, add water 100mL, It was extracted with EA (100 mL*3), concentrated and recrystallized with ethanol to obtain 10.94 g of a yellow solid product with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com