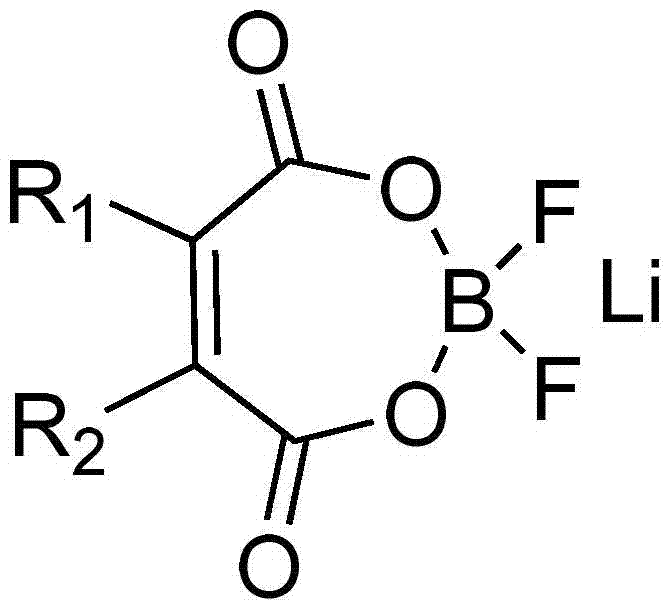
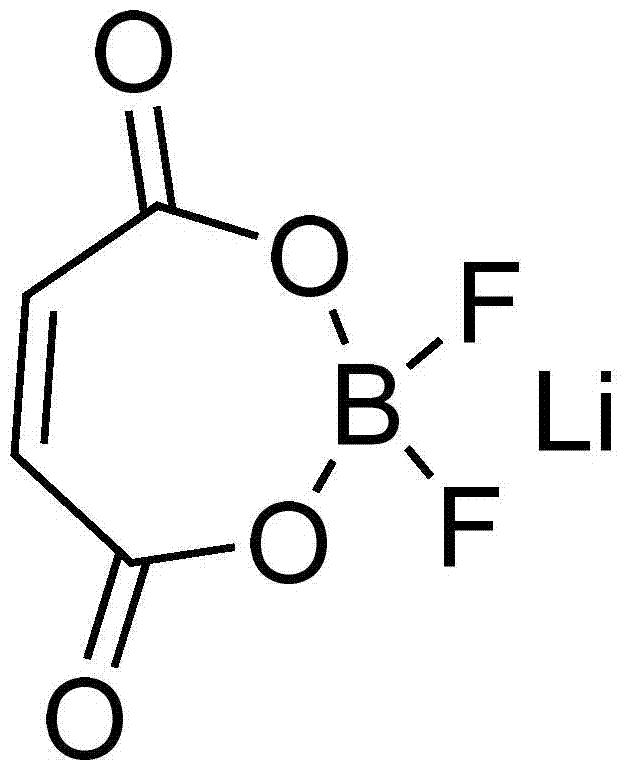
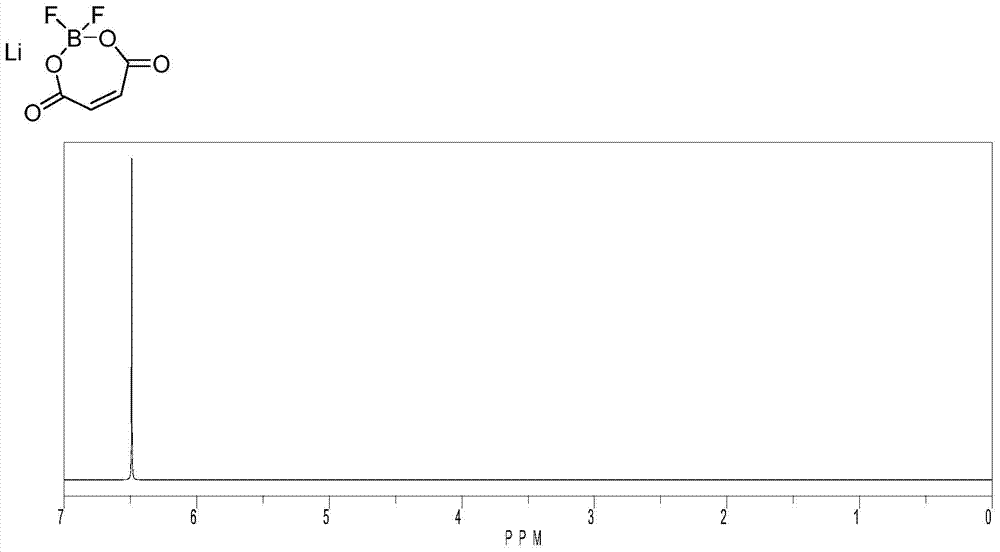
Lithium maleate difluoroborate and electrolyte for lithium secondary battery containing the same
A technology of lithium maleate difluoroborate and lithium secondary battery, which is applied in the application field of lithium secondary battery and lithium secondary battery electrolyte, and can solve the problem of poor low-temperature conductivity, low decomposition temperature of lithium hexafluorophosphate, and limited lithium Ion battery applications and other issues to achieve the effect of improving cycle life and cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Lithium maleate difluoroborate was prepared from boron trifluoride-diethyl ether solution, lithium maleate, trimethylmethoxysilane and maleic acid as raw materials.
[0043]In a reaction flask equipped with a magnetic stirrer, 127.9 g of lithium maleate was added, 500 mL of acetonitrile was added, stirring was started, and 288 g of boron trifluoride-ether solution was slowly added dropwise thereto. After the dropwise addition was completed, the stirring was continued. After all the solid materials were dissolved, 116.07 g of maleic acid was added, and 208.4 g of trimethylmethoxysilane was slowly added dropwise, and the mixture was reacted at 20° C. for 10 hours. To fully convert the raw materials. Stop heating and cool to room temperature naturally. The solvent and by-products were distilled off under reduced pressure. The reaction solution was concentrated and crystallized, and filtered under nitrogen protection. The filter cake was vacuum-dried at a temperature of ...
Embodiment 2
[0046] Lithium maleate difluoroborate was prepared from boron trifluoride-diethyl ether solution, lithium fluoride, trimethylethoxysilane and maleic acid.
[0047] In a reactor equipped with a heating and magnetic stirring device, 25.9 g of lithium fluoride and 500 mL of dimethyl carbonate were added, stirring was started, and 144.2 g of boron trifluoride-diethyl ether solution was slowly added dropwise therein. After the dropwise addition is completed, continue to stir until all solid materials are dissolved. Add 116.07 g of maleic acid, slowly add 236.5 g of trimethylethoxysilane at room temperature, heat to 80° C., and react for 2 hours. To fully convert the raw materials. Stop heating and cool to room temperature naturally.
[0048] The reaction solution was concentrated and crystallized, and filtered under nitrogen protection. The filter cake was vacuum-dried at a temperature of 60-80° C. for 48 hours. Finally, 135.8 g of white powdery solids were obtained, with a yie...
Embodiment 3
[0051] Lithium maleate difluoroborate was prepared from boron trifluoride-acetonitrile solution, lithium fluoride and diethyl aluminum monochloride maleate.
[0052] In a reactor equipped with a heating and magnetic stirring device, 25.94 g of lithium fluoride and 1000 mL of diethyl carbonate were added, stirring was started, and 141.9 g of boron trifluoride-ether solution was slowly added dropwise therein. After the dropwise addition, continue to stir until all solid materials are dissolved, slowly add 241g of diethylaluminum monochloride at room temperature, add 116.07g of maleic acid, heat to 60°C, and react for 4 hours. To fully convert the raw materials. Stop heating and cool to room temperature naturally.
[0053] The reaction solution was concentrated and crystallized, and filtered under nitrogen protection. The filter cake was vacuum-dried at a temperature of 60-80° C. for 48 hours. Finally, 169.5 g of white powdery solid was obtained with a yield of 99%.
[0054] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com