Supercritical CO2 dyeing special-purpose azo-type active disperse dye
A reactive disperse dye, azo-type technology, applied in the direction of reactive dyes, azo dyes, monoazo dyes, etc., can solve the problem of loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Weigh 0.002mol 2-chloro-4-nitroaniline, add 50mL polyethylene glycol 200 and 50mL deionized water, place it in an ultrasonic cleaner to shake and dissolve, then add 0.02mol concentrated hydrochloric acid to the solution And transfer it to a three-necked flask equipped with a magnetic stirring bar and a thermometer, in an ice-water bath, and control the temperature at 0-5°C. After stirring evenly, slowly add 0.0022mol of sodium nitrite (dissolved in 5mL of deionized water) to the reaction solution dropwise. After 2.5 hours of reaction, add 0.0002mol of urea (dissolved in 5mL of deionized water), and stir for 15 minutes to obtain a yellow , Clear diazonium salt solution.
[0019] (2) Measure 0.0022mol m-toluidine (add 5mL polyethylene glycol 200 to dilute as a diluent), slowly drop it into the above-mentioned diazonium salt solution, and adjust the pH value to about 6 with 30% NaOH aqueous solution , keep the temperature at 0-5°C, and finish the reaction after 4h. T...
Embodiment 2
[0028] The supercritical CO provided by this embodiment 2 Dyeing special-purpose azo-type reactive disperse dyes, the steps of its synthetic method are the same as in Example 1, wherein m-toluidine in step (2) is changed to 3-methoxyaniline, and the yield of the product obtained is 97.55%. In step (3), weigh 0.001 mol of the product in step (2), add 50 mL of 1,4-dioxane and 50 mL of deionized water, and finally obtain a crude reactive disperse dye with a yield of 94.73%. In the step (4), the crude product of the reactive disperse dye obtained in the step (3) is purified.
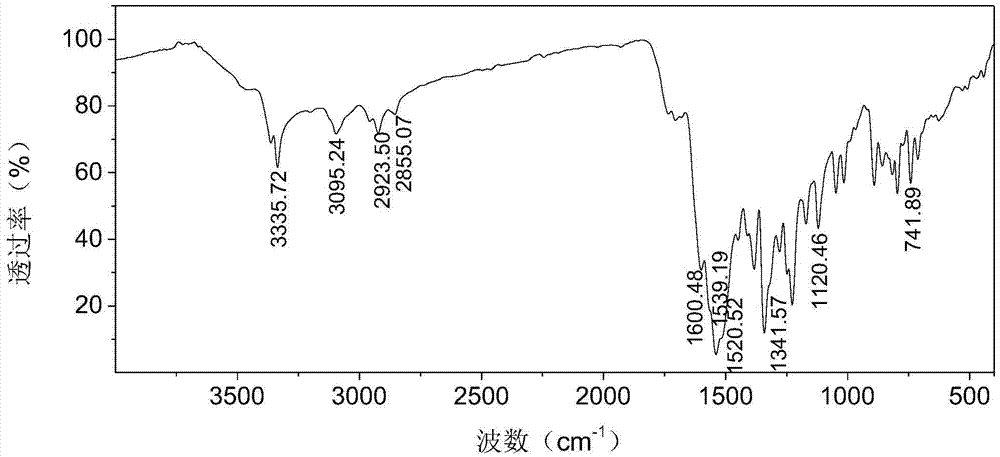
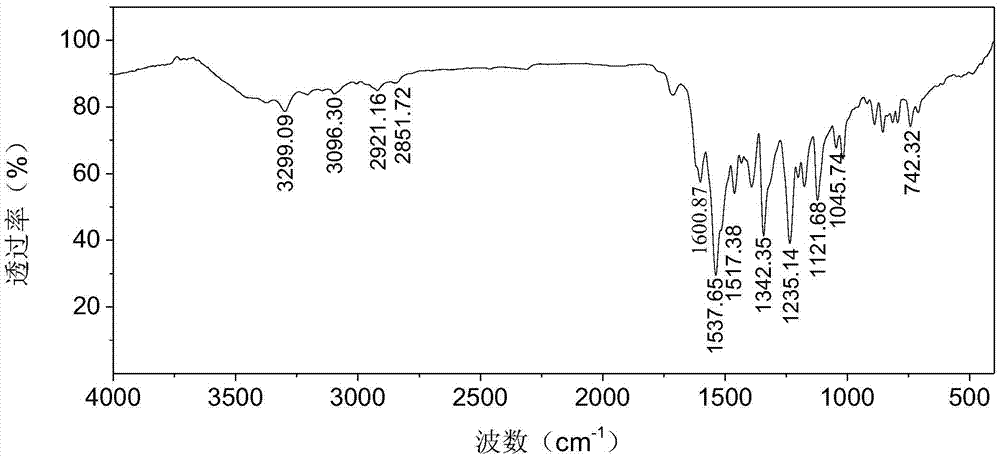
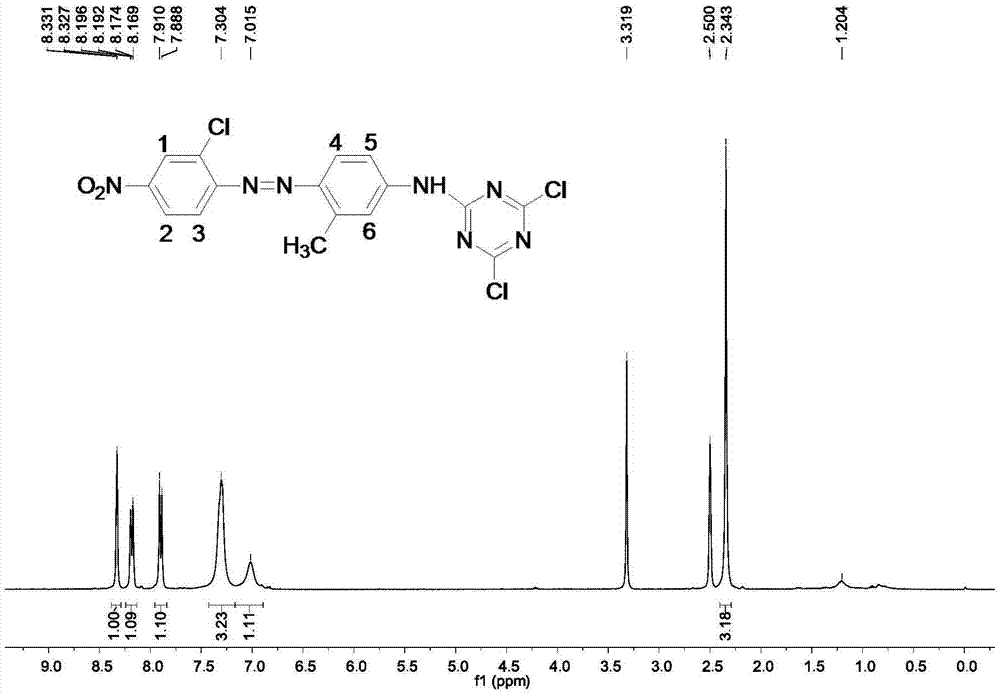
[0029] Using Fourier transform infrared spectroscopy, hydrogen nuclear magnetic resonance spectroscopy (400MHz, DMSO-d 6 ) and UV-visible absorption spectroscopy and other testing techniques to characterize the structure of the purified product obtained in this example, the results are as follows figure 2 , 4 , 5 shown.
[0030] figure 2 shows that at 3299.09cm -1 There is N-H stretching vibration pe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com