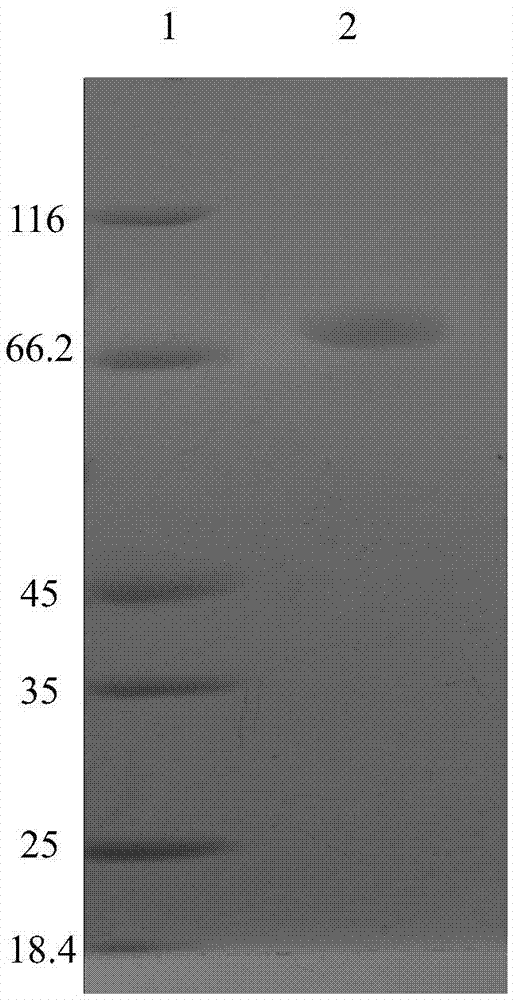
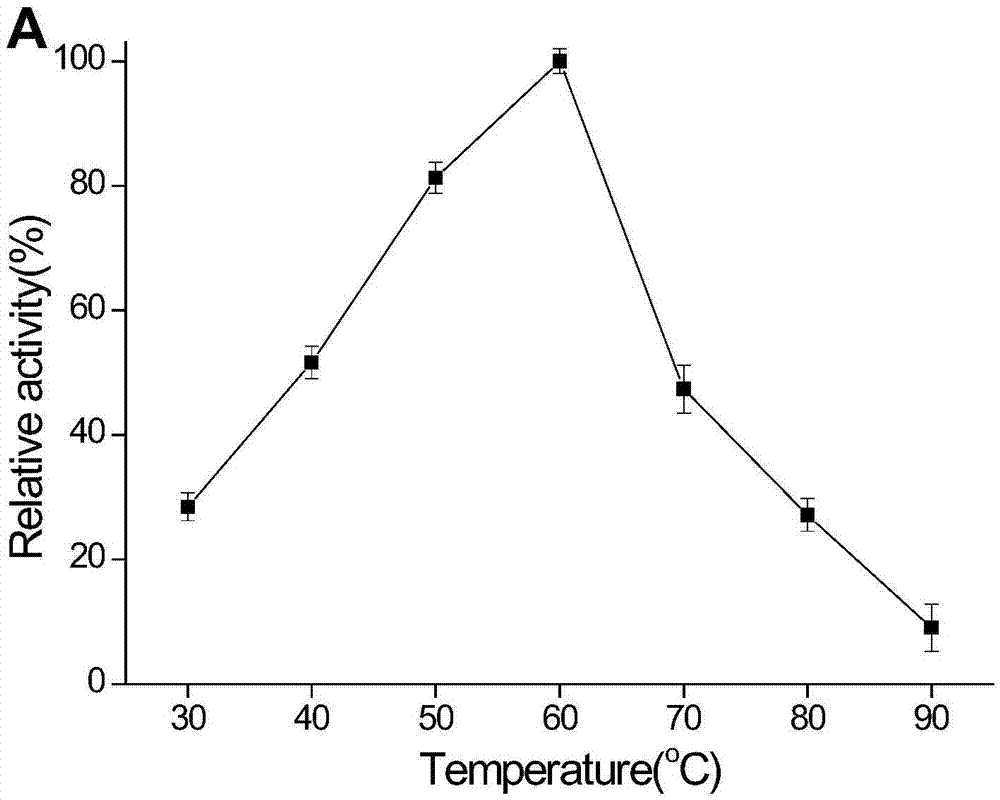
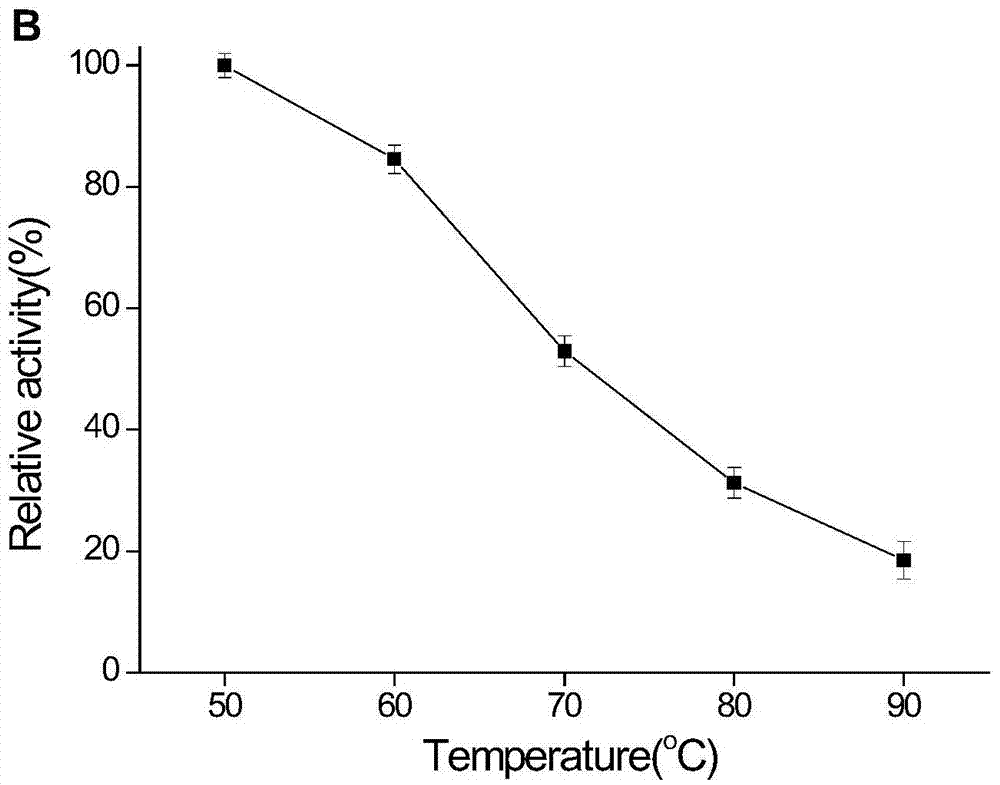
Novel heat-resistant dual-function glucoside hydrolase MtCel2 and encoding sequence and application thereof
A technology of glycoside hydrolase and coding sequence, applied in thermostable bifunctional glycoside hydrolase MtCel2 and its coding sequence and application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Isolation and Identification of Myceliophthorathermophila Myceliophthorathermophila
[0021] (1) Specimen collection: collected from compost.
[0022] (2) Separation and cultivation: 0.5 g of the collected specimens were placed on a PDA plate and cultured at 50° C. for 3 days before separation and purification. Operation steps refer to Cooney and Emerson (1964) literature.
[0023] (3) Identification: Refer to the documents of Cooney and Emerson (1964) and LaTouche (1950).
Embodiment 2
[0024] Embodiment 2: Cloning of thermostable glycoside hydrolase gene MtCel2
[0025] (1) Extraction of total RNA from Myceliophthorathermophila: refer to the instructions of the Trizol kit.
[0026] (2) Synthesis of the first strand of cDNA: according to the instructions of the TaKaRaRNAPCRkit (AMV) Ver3.0 kit from Takara Company: take 1-2 μg of total RNA, add RNaseFreeddH 2 From 0 to 9.5 μL, denature the RNA sample at 75°C for 5 min, immediately cool it in an ice bath for 5 min, then centrifuge slightly, and add the following components in sequence in the ice bath: 10 mmol / LdNTP Mixture 2 μL, 10×RTBuffer (Mg 2+ )2μL, 25mmol / LMgCl 2 4 μL, Oligod (T)-Adaptor Primer 1 μL, RNase Inhibiter 0.5 μL, AMVReverse Transcriptase 1 μL (Final Volume 20 μL), after mixing the reaction solution, let it stand at room temperature for 10 minutes, then incubate at 42°C for 60 minutes, and then boil for 5 minutes to inactivate the reverse transcriptase. Add 180 μL DEPC-treated ddH 2 O, dilute ...
Embodiment approach 3
[0046] Embodiment 3: Construction of expression vector
[0047] (1) Design expression primers according to the nucleotide sequence of the isolated MtCel2 gene, respectively introduce EcorI and NotI restriction sites at the 5' end of the primers, and introduce 6 histidine sequences into the downstream primers as purification tags:
[0048] Upstream primer: 5'-CCG GAATTC GCGCCCACGAACACT-3' (EcorI)
[0049] Downstream primer: 5'-TT GCGGCCGC TAGTGGTGGTGGTGGTGGTGCTCGCACTTGCGCCAAG-3'(NotI) (histidine sequence)
[0050] (2) Extraction of total RNA from Myceliophthora thermophila: extraction with Trizol reagent.
[0051] (3) Synthesize the first strand of cDNA by reverse transcription: take 2 μg total RNA, add 4 μL of 5× reaction buffer, 2 μL of 10 mMdNTP, 0.5 μL of ribonuclease inhibitor (40-200u / μL), primer oligodT (1 μg / μL) 1 μL, 2 μL of reverse transcriptase (10u / μL), react at 42°C for 60 minutes, then stop the reaction at 85°C for 10 minutes, and dilute to 200 μL.
[0052] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com