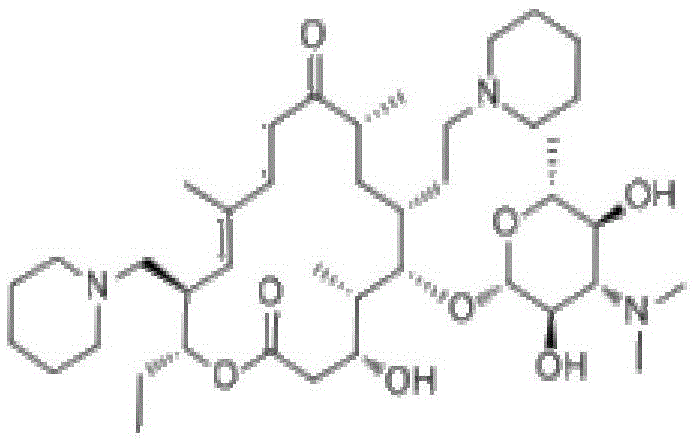
Detection method of Tildipirosin content
A technology of tediloxine and detection method, which is applied in the field of chemical analysis, can solve the problems of high difficulty, high requirements, and difficulty in quality control of tediloxine, and achieves the effects of accurate method, high sensitivity and good repeatability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of new samples of tedrol:
[0022] Preparation of the standard solution: Weigh 50 mg of Tedirosin standard, weigh it accurately, put it in a 50ml volumetric flask, add mobile phase to dissolve and set the volume to the mark, and shake well. Take the solution with a 1ml syringe and filter it through a 0.45um organic filter head to obtain a standard solution.
[0023] Preparation of the test solution: Weigh 50 mg of Tedirosin sample, accurately weigh it, place it in a 50 ml volumetric flask, add an appropriate amount of mobile phase, dissolve it and set the volume to the mark, and shake well. Take the solution with a 1ml syringe and filter it through a 0.45um organic filter head to obtain the test solution.
[0024] Five copies of each of the standard solution and the test solution were prepared.
[0025] Chromatographic analysis conditions:
[0026] Shimadzu LC-20A liquid chromatograph, WatersC18 column (250*4.6, 5um);
[0027] Mobile phase: methanol: ace...
Embodiment 2
[0049] Samples and standard preparations are the same as in Example 1.
[0050] Chromatographic analysis conditions:
[0051] Shimadzu LC-20A liquid chromatograph, WatersC8 column (150*4.6, 3.5um);
[0052] Mobile phase: methanol: acetonitrile: 0.05mog / L potassium dihydrogen phosphate solution (pH6.50) = (40:25:35);
[0053] Flow rate: 0.8ml / min;
[0054] Detection wavelength: 290nm;
[0055] Injection volume: 20ul;
[0056] Detector: UV detector;
[0057] Column oven: 30°C.
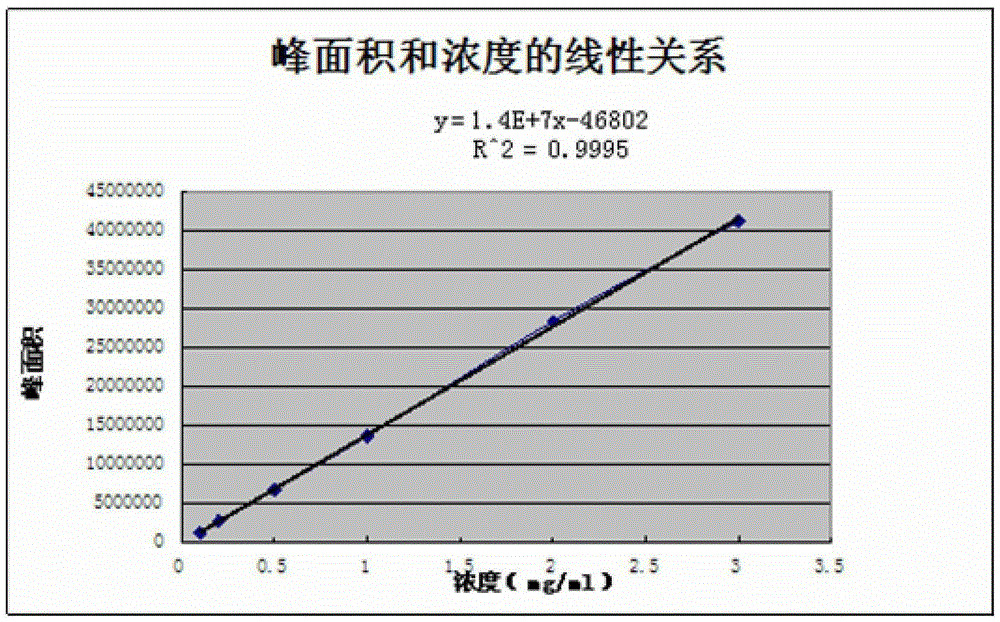
[0058] According to the above-mentioned chromatographic conditions, inject samples respectively to all standard substances and test substances, such as figure 1 As shown, the peak area obtained by injecting 5 samples of the standard solution respectively was 12545262, and the RSD was 0.21%; the average value of the peak area obtained by the 5 samples of the test solution was 10201259, and the RSD was 0.22%.
[0059] According to the formula:
[0060] The content of tadiroxin: a=A sample M standar...
Embodiment 3
[0063] Samples and standard preparations are the same as in Example 1.
[0064] Chromatographic analysis conditions: Shimadzu LC-20A liquid chromatograph, WatersC8 chromatographic column (150*4.6, 3.5um);
[0065] Mobile phase: methanol: acetonitrile: 0.05mog / L potassium dihydrogen phosphate solution (pH7.50) = (40:30:40);
[0066] Flow rate: 1.2ml / min;
[0067] Detection wavelength: 270nm;
[0068] Injection volume: 10ul;
[0069] Detector: UV detector;
[0070] Column oven: 40°C.
[0071] According to the above-mentioned chromatographic conditions, inject samples respectively to all standard substances and test substances, such as figure 1 As shown, the peak area obtained by injecting 5 samples of the standard solution was 12135495 respectively, and the RSD was 0.10%; the average value of the peak area obtained by the 5 samples of the test solution was 10594411, and the RSD was 0.09%.
[0072] According to the formula:
[0073] The content of tadiroxin: a=A sample M sta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com