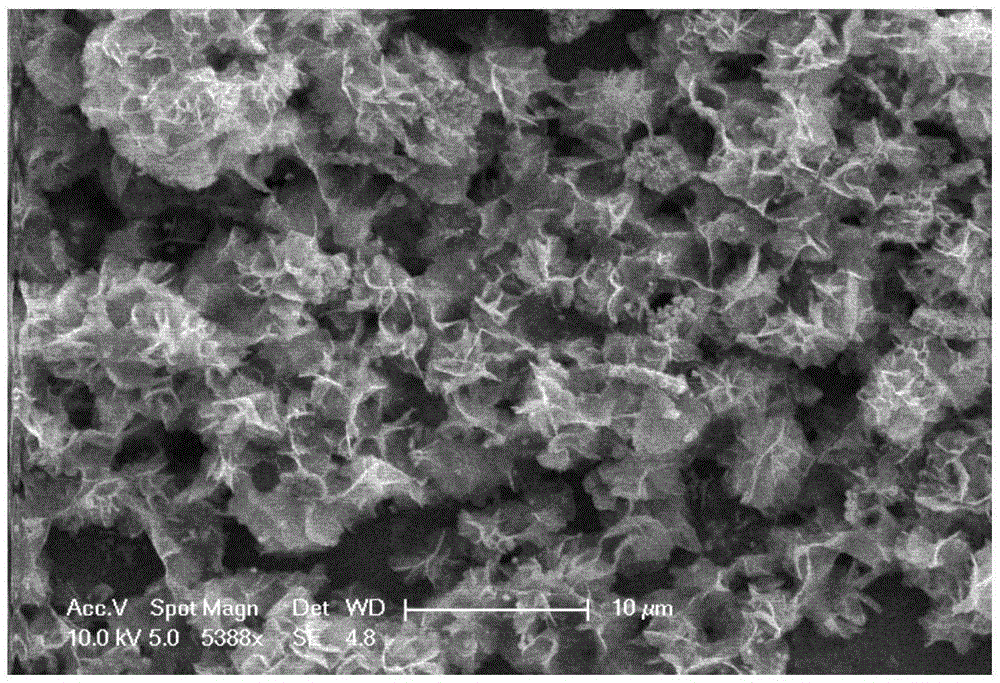
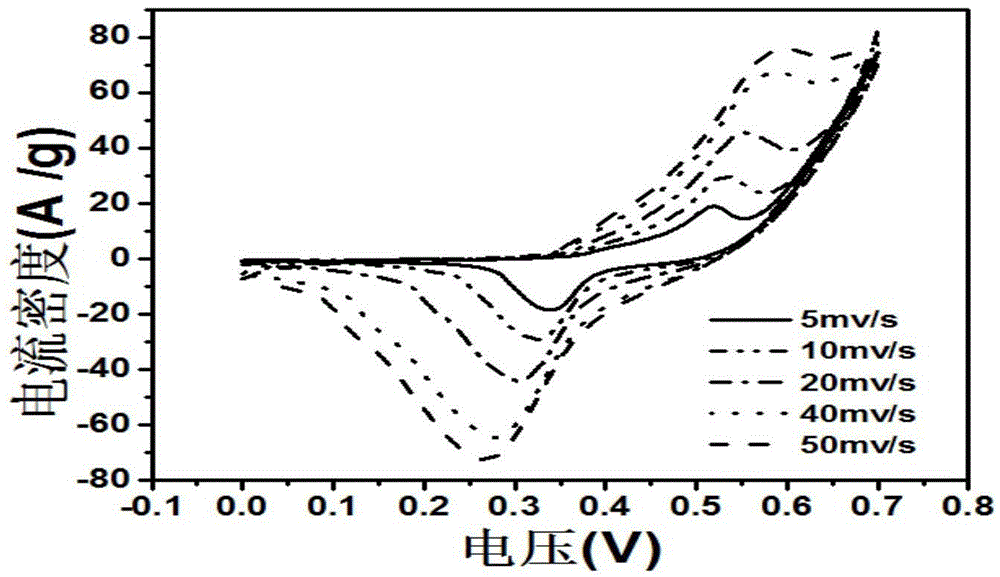
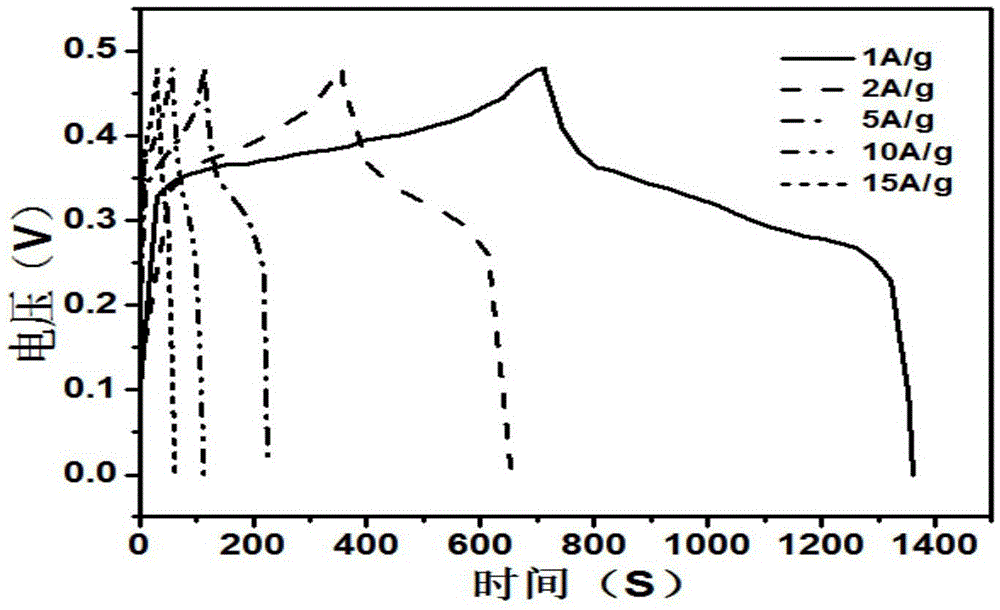
Preparation method of flower-like nickel sulfide material and application of flower-like nickel sulfide material in super capacitor
A nickel sulfide, flower-shaped technology, applied in the application of supercapacitors, flower-shaped nickel sulfide materials, the field of preparation of flower-shaped nickel sulfide materials, can solve the problem of decreased electrical conductivity and specific capacitance, poor stability of supercapacitors, The problem of large changes in electrode volume expansion has achieved the effect of improving cycle stability, improving cycle stability, and increasing specific capacitance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Dissolve the nickel source precursor nickel acetate in a certain amount of deionized water, then add a certain amount of ethanol, and ultrasonically disperse for 30 minutes at a temperature of 15-40°C, a power of 100-200W, and a frequency of 15-20kHz. -40min, then add thiourea, the precursor of the sulfur source, and a certain amount of F127, and ultrasonically disperse for 30-40min at a temperature of 15-40°C, a power of 100-200W, and a frequency of 15-20kHz to obtain a reaction solution;
[0029] Wherein the mass ratio of the nickel acetate that adds in the above-mentioned mixed solution and thiourea is 1:0.92, the mass ratio of the F127 that adds and nickel acetate is 5:274, the volume ratio of the ethanol that adds in the mixed solution and deionized water is 1: 5;
[0030] (2), transfer the reaction liquid obtained in step (1) into a hydrothermal kettle for solvothermal reaction, control the temperature at 180°C for 24 hours, wash the obtained reaction liquid w...
Embodiment 2
[0037] (1) Dissolve the nickel source precursor nickel acetate in a certain amount of deionized water, then add a certain amount of ethanol, and ultrasonically disperse for 30 minutes at a temperature of 15-40°C, a power of 100-200W, and a frequency of 15-20kHz. -40min, then add thiourea, the precursor of the sulfur source, and a certain amount of F127, and ultrasonically disperse for 30-40min at a temperature of 15-40°C, a power of 100-200W, and a frequency of 15-20kHz to obtain a reaction solution;
[0038] Wherein the mass ratio of the nickel acetate that adds in the above-mentioned mixed solution and thiourea is 1:1.53, the mass ratio of the F127 that adds and nickel acetate is 10:274, the ethanol that adds in the mixed solution and the volume ratio of deionized water are 1: 5;
[0039] (2), move the reaction liquid obtained in step (1) into a hydrothermal kettle for solvothermal reaction, and carry out the reaction at a temperature of 180°C for 24 hours, and wash the obta...
Embodiment 3
[0043] (1) Dissolve the nickel source precursor nickel acetate in a certain amount of deionized water, then add a certain amount of ethanol, and ultrasonically disperse for 30 minutes at a temperature of 15-40°C, a power of 100-200W, and a frequency of 15-20kHz. -40min, then add thiourea, the precursor of the sulfur source, and a certain amount of F127, and ultrasonically disperse for 30-40min at a temperature of 15-40°C, a power of 100-200W, and a frequency of 15-20kHz to obtain a reaction solution;
[0044] Wherein the mass ratio of the nickel acetate that adds in the above-mentioned mixed solution and thiourea is 1:0.92, the mass ratio of the F127 that adds and nickel acetate is 10:274, the volume ratio of the ethanol that adds in the mixed solution and deionized water is 1: 3;
[0045] (2), move the reaction liquid obtained in step (1) into a hydrothermal kettle for solvothermal reaction, control the temperature at 180°C for 24 hours, wash the obtained reaction liquid with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com